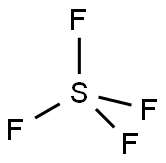
Is SF4 Polar or Nonpolar?
Sulfur tetrafluoride (SF4) is a polar molecule. It is made up of four fluorine (F) atoms bonded to a sulfur (S) atom. Due to the electronegativity difference between S and F atoms, each S-F bond in the SF4 molecule is polar.
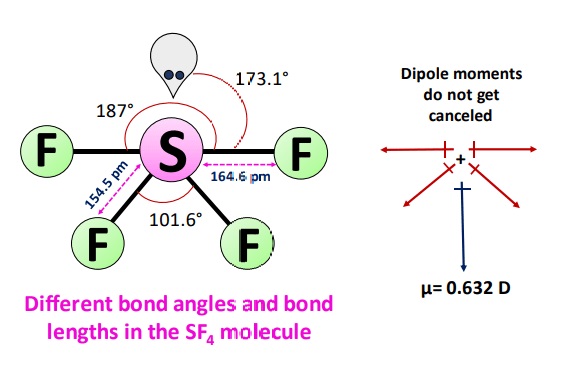
There are three different bond angles in the SF4 molecule i.e., 101.6°,173.1° and 187°. As the shape of the molecule is like a see-saw, two fluorine atoms can cancel out each other’s dipole moment, but the rest two can’t due to the electrons’ arrangement. The overall electron cloud is not evenly balanced in this asymmetric seesaw shape.
Fluorine is more electronegative than Sulfur due to which the overall charge distribution of a molecule is uneven resulting in a polar molecule and give a net dipole moment (µ) value of 0.632 D.
So yes, SF4 is polar.
);You may like
Lastest Price from Sulfur tetrafluoride manufacturers

US $15.00-10.00/KG2021-08-12
- CAS:
- 7783-60-0
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton