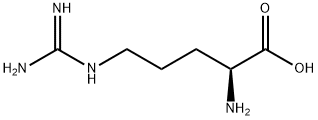
L(+)-Arginine: Function, source and pharmacodynamics
General description
−), the amino group is protonated (−NH3+), and the guanidino group is also protonated to give the guanidinium form (-C-(NH2)2+), making arginine a charged, aliphatic amino acid.[1] L(+)-Arginine is the precursor for the biosynthesis of nitric oxide. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. Its appearance is as follows:

Figure 1 Appearance of L(+)-Arginine.
Function
L(+)-Arginine plays an important role in cell division, wound healing, removing ammonia from the body, immune function,[2] and the release of hormones.[3] It is a precursor for the synthesis of nitric oxide (NO), making it important in the regulation of blood pressure. L(+)-Arginine's side chain is amphipathic, because at physiological pH it contains a positively charged guanidinium group, which is highly polar, at the end of a hydrophobic aliphatic hydrocarbon chain. Because globular proteins have hydrophobic interiors and hydrophilic surfaces, L(+)-Arginine is typically found on the outside of the protein, where the hydrophilic head group can interact with the polar environment, for example taking part in hydrogen bonding and salt bridges.[4] For this reason, it is frequently found at the interface between two proteins. The aliphatic part of the side chain sometimes remains below the surface of the protein.[4]
L(+)-Arginine is the immediate precursor of NO, an important signaling molecule which can act as a second messenger, as well as an intercellular messenger which regulates vasodilation, and also has functions in the immune system's reaction to infection. L(+)-Arginine is also a precursor for urea, ornithine, and agmatine; is necessary for the synthesis of creatine; and can also be used for the synthesis of polyamines (mainly through ornithine and to a lesser degree through agmatine, citrulline, and glutamate.) The presence of asymmetric dimethylarginine (ADMA), a close relative, inhibits the nitric oxide reaction; therefore, ADMA is considered a marker for vascular disease, just as L(+)-Arginine is considered a sign of a healthy endothelium.
Source
L(+)-Arginine is a conditionally essential amino acid in humans and rodents, as it may be required depending on the health status or lifecycle of the individual. For example, while healthy adults can supply their own requirement for arginine, immature and rapidly growing individuals require L(+)-Arginine in their diet, and it is also essential under physiological stress, for example during recovery from burns, injury, and sepsis, or when the small intestine and kidneys, which are the major sites of L(+)-Arginine biosynthesis, have been damaged. It is, however, an essential amino acid for birds, as they do not have a urea cycle. For some carnivores, for example cats, dogs and ferrets, L(+)-Arginine is essential, because after a meal, their highly efficient protein catabolism produces large quantities of ammonia which need to be processed through the urea cycle, and if not enough arginine is present, the resulting ammonia toxicity can be lethal.[5] This is not a problem in practice, because meat contains sufficient L(+)-Arginine to avoid this situation.[5]
Pharmacodynamics
About 5 g of L(+)-Arginine is ingested each day in a normal Western diet. L(+)-Arginine plasma levels are not significantly reduced in most disease conditions, except end-stage renal failure during hemodialysis treatment. Nonetheless, intravenous or dietary (oral) administration of relatively large doses of L(+)-Arginine has been shown to result in enhanced NO formation in subjects with impaired endothelial function at baseline. In several controlled clinical trials, long-term administration of L(+)-Arginine has been shown to improve the symptoms of cardiovascular disease. However, in other trials L(+)-Arginine was not beneficial, and in a recent study, the authors reported higher mortality of subjects receiving L(+)-Arginine than those receiving placebo.[6]
References
[1]Glasel et al. (1995). Introduction to Biophysical Methods for Protein and Nucleic Acid Research. Academic Press. p. 456. ISBN 978-0-08-053498-5.
[2]Mauro et al. (2015). The Metabolic Challenges of Immune Cells in Health and Disease. Frontiers Media SA. p. 17. ISBN 9782889196227.
[3]Stechmiller et al. (2005). Arginine supplementation and wound healing. Nutrition in Clinical Practice. 20 (1): 52–61.
[4]Barnes. (2007). Bioinformatics for Geneticists: A Bioinformatics Primer for the Analysis of Genetic Data. John Wiley & Sons. p. 326. ISBN 9780470026199.
[5]Wortinger et al. (2015). Nutrition and Disease Management for Veterinary Technicians and Nurses. John Wiley & Sons. p. 232. ISBN 978-1-118-81108-5.
[6]Rainer. (2007). The Pharmacodynamics of L-Arginine. The Journal of Nutrition, 137(6): 1650S–1655S );You may like
Related articles And Qustion
See also
Lastest Price from L(+)-Arginine manufacturers

US $0.00/Kg/Drum2024-04-30
- CAS:
- 74-79-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200mt
US $0.00-0.00/kg2024-04-30
- CAS:
- 74-79-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1T+