Levulinic acid: properties, applications and safety
General Description
Levulinic acid is a naturally occurring, versatile compound with several notable properties. It finds applications in various industries, including pharmaceuticals, agriculture, cosmetics, and materials manufacturing, highlighting its diverse functional uses and industrial significance. However, safety precautions should be taken when handling the substance due to its potential hazards such as oral toxicity, skin irritation, and eye irritation. Proper safety measures can mitigate potential risks. Overall, the wide-ranging applications of levulinic acid and its importance across industries underscore its significance as a valuable compound with diverse functional uses and industrial significance.

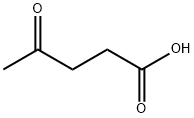
Figure 1. Levulinic acid
Properties
Levulinic acid is a naturally occurring compound with the molecular formula C5H8O3 and a molecular weight of 116.11 g/mol. It is a straight-chain saturated fatty acid and an oxopentanoic acid, featuring an oxo group in the 4-position. This acid serves as a plant metabolite and is produced by Escherichia coli (strain K12, MG1655). Levulinic acid can be found in Dendronephthya hemprichi as well. From a chemical perspective, levulinic acid has several notable properties. It is a yellow to brown liquid that may congeal, characterized by a mild caramellic odor. The compound has a melting point of 30-33 °C and a boiling point of 245.00 to 246.00 °C at 760.00 mm Hg. It is soluble in water, alcohol, and oil, and its density ranges from 1.136 to 1.147 (20°C). The compound's logP value is -0.49, and it has a refractive index of 1.439-1.445. Additionally, it has a pKa value of 4.64 (at 18°C). In terms of its structural properties, levulinic acid comprises eight heavy atoms, with a topological polar surface area of 54.4Ų. It contains one hydrogen bond donor and three hydrogen bond acceptors, along with three rotatable bonds. These properties make levulinic acid a versatile compound with potential applications in various fields, including the pharmaceutical, agricultural, and chemical industries. 1
Applications
Levulinic acid, a versatile compound, finds applications in various industries. It is used in the production of nylon, synthetic rubber, plastics, and pharmaceuticals, showcasing its significance in industrial processes. Additionally, it serves as a flavoring agent in food, animal feeds, and cosmetic products, highlighting its diverse functional uses. In the agricultural sector, levulinic acid is utilized as a flavoring agent for animal feeds, demonstrating its role in enhancing palatability and nutritional value. Its presence in farming as a feed additive underscores its importance in the food chain and animal husbandry practices. Furthermore, the compound is classified under lipid maps as an oxo fatty acid, emphasizing its relevance in lipid-related research and applications. In consumer products, levulinic acid serves as an intermediate and laboratory chemical, indicating its potential applications in the development of various consumer goods. Additionally, it is employed in skin conditioning formulations within the cosmetics industry, showcasing its role in personal care and beauty products. Overall, the wide-ranging applications of levulinic acid across industries, including food, agriculture, cosmetics, and materials manufacturing, underscore its importance as a valuable compound with diverse functional uses and industrial significance. 2
Safety
Safety information for levulinic acid indicates potential hazards and precautions to be taken when handling the substance. According to the GHS classification, levulinic acid is classified as an irritant. It may cause harm if swallowed, skin irritation, and serious eye irritation. Precautionary measures such as proper personal protective equipment (PPE) and hygiene practices should be followed when dealing with this compound. Toxicological information reveals acute effects of levulinic acid in animal studies. The LD50 (lethal dose 50%) for oral ingestion in rats is 1850 mg/kg, and for intraperitoneal injection in mice, it is 450 mg/kg. However, the LD50 for skin exposure in rabbits is reported to be greater than 5 gm/kg, indicating lower toxicity through dermal contact. In summary, while levulinic acid poses certain hazards such as oral toxicity, skin irritation, and eye irritation, proper safety measures can mitigate potential risks. 3
Reference
1. Levulinic acid. National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 11579.
2. Dionisio KL, Phillips K, Price PS, Grulke CM, Williams A, Biryol D, Hong T, Isaacs KK. The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Sci Data. 2018 Jul 10;5:180125.
3. 4-oxovaleric acid. European Chemicals Agency, EC / List no. 204-649-2.
);You may like
Related articles And Qustion
Lastest Price from Levulinic acid manufacturers

US $50.00/kg2024-04-28
- CAS:
- 123-76-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000kg/Month

US $30.00/kg2024-04-28
- CAS:
- 123-76-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000kg