Linolenic Acid: A Closer Look at Its Synthesis, Composition, and Regulatory Aspects
Introduction
Linolenic acid, also known as alpha-linolenic acid (ALA), is a crucial polyunsaturated omega-3 fatty acid that plays an integral role in human physiology and nutrition. Despite being an essential nutrient, which means it must be ingested because the human body cannot synthesize it, linolenic acid is a cornerstone in understanding the dietary impacts on cardiovascular health, inflammation, and cellular function. Its significance extends to both preventive health measures and therapeutic applications, making it a focus of ongoing research and discussion in the scientific community[1].

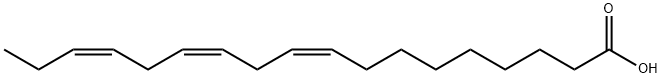
Figure 1 Characteristics of Linolenic acid
Synthesis Methods
In the natural world, linolenic acid is synthesized by plants and certain algae via a series of enzymatic reactions that elongate and desaturate shorter-chain fatty acids. These reactions are facilitated by a family of enzymes known as fatty acid desaturases, which introduce double bonds into fatty acyl chains. Specifically, the synthesis of linolenic acid involves the conversion of linoleic acid (18:2 omega-6) through the action of the omega-3 desaturase, which adds a double bond to create the three characteristic cis double bonds of linolenic acid at the 9th, 12th, and 15th carbon atoms.
Industrially, linolenic acid can be synthesized through chemical methods, such as catalytic desaturation, or via the use of microorganisms that produce omega-3 fatty acids through fermentation processes. These microbial processes involve genetically modified bacteria or yeast, which are capable of converting sugars into fatty acids, including linolenic acid, under controlled conditions.
Main Constituents
Chemically, linolenic acid consists of a chain of 18 carbons with three cis double bonds, which are located at the 9th, 12th, and 15th positions. This unique structure not only defines its liquid state at room temperature but also enhances its chemical reactivity. Linolenic acid’s role in the body is multifaceted; it is a precursor to eicosanoids, which are signaling molecules that play critical roles in inflammation and immunity[2].
Additionally, linolenic acid is incorporated into cell membranes, altering their fluidity and functionality. The presence of double bonds in linolenic acid contributes to its susceptibility to oxidative damage, which is a significant consideration in food processing and storage.
Usage Limits
The intake of linolenic acid is regulated by health authorities worldwide due to its potent biological effects. For instance, the FDA suggests a daily intake of up to 1.6 grams for men and 1.1 grams for women as adequate, while EFSA provides similar guidelines with a slightly higher recommended intake due to its cardiovascular benefits. However, these limits are set to prevent the potential negative effects of excessive consumption, which can include increased blood coagulation times and possible impacts on immune response.
Toxicity
Although linolenic acid is generally considered safe within the recommended consumption levels, its high degree of unsaturation makes it prone to oxidation, which can lead to the formation of lipid peroxides. These oxidation products can be harmful if ingested in large quantities, potentially leading to cellular damage and contributing to conditions such as atherosclerosis. The stability of linolenic acid is thus a critical factor in both dietary applications and industrial usage, necessitating the addition of antioxidants in products rich in linolenic acid to prevent degradation.
Conclusion
Linolenic acid is a fundamental component in the diet and serves a myriad of roles in human health. Its importance in reducing inflammatory processes and contributing to the structural integrity of cell membranes makes it a focal point of research in nutritional biochemistry. For professionals in the chemical and health sciences, understanding the synthesis, structure, regulated limits, and potential risks associated with linolenic acid is essential. As dietary guidelines evolve and more is learned about the intricate roles of fatty acids, the profile of linolenic acid will continue to be a key area of scientific inquiry and regulatory review.
References
[1]Stark A H, Crawford M A, Reifen R. Update on alpha-linolenic acid[J]. Nutrition Reviews, 2008, 66(6): 326-332.
[2]Burdge G C. Metabolism of α-linolenic acid in humans[J]. Prostaglandins, leukotrienes and essential fatty acids, 2006, 75(3): 161-168.
);You may like
Related articles And Qustion
Lastest Price from Linolenic acid manufacturers

US $0.00/kg2024-04-22
- CAS:
- 463-40-1
- Min. Order:
- 1kg
- Purity:
- 40% 60% 95% 98%
- Supply Ability:
- 3000 kg
US $0.00/kg2024-03-28
- CAS:
- 463-40-1
- Min. Order:
- 25kg
- Purity:
- 80%, 85%
- Supply Ability:
- Inquiry