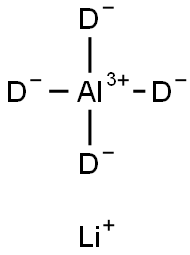Lithium aluminum deuteride: Synthesis Procedure, Electrochemical lithium storage properties and mechanism
General Description
The synthesis of Lithium aluminum deuteride involves ball milling LiAlH4 with LiH to achieve approximately 93% purity. Electrode material is prepared through low-energy ball milling with acetylene black. Electochemical evaluation shows Lithium aluminum deuteride delivers 1729 mAh/g during the first discharge cycle, but only recharges about 29.4% of this capacity due to irreversible reactions. The storage mechanism involves phase transitions, forming LiH and Al during discharge, with LiAl emerging. XRD and FTIR analyses reveal structural changes and electrolyte reactions. In summary, Lithium aluminum deuteride exhibits high initial capacity but low coulombic efficiency due to irreversible processes during cycling.

Figure 1. Lithium aluminum deuteride
Synthesis procedure
The synthesis procedure of Lithium aluminum deuteride (Li3AlH6) involves ball milling of LiAlH4 with LiH, as shown in Figure 1a. The process lasts for 24 hours and during this high-energy ball milling, approximately 0.44 wt% of hydrogen is released. By using the synthesis equation, it is determined that this amount of hydrogen corresponds to around 7% of Li3AlH6. Thus, a purity of approximately 93% Li3AlH6 is obtained. To prepare the electrode material, low-energy ball milling is performed using Lithium aluminum deuteride and acetylene black in a weight ratio of 85:15. The resulting electrode material is found to be a mixture of Lithium aluminum deuteride and acetylene black, as confirmed by various analytical techniques. X-ray diffraction (XRD) pattern, Fourier Transform Infrared Spectrometer (FTIR), and Raman spectra analyses were conducted (refer to Figure S1) to determine the composition and characteristics of the electrode material. In summary, the synthesis procedure of Lithium aluminum deuteride involves ball milling LiAlH4 with LiH, followed by low-energy ball milling with acetylene black to obtain the desired electrode material. The purity of Li3AlH6 is calculated based on the amount of hydrogen released during the high-energy ball milling process. 1
Electrochemical lithium storage properties
The electrochemical lithium storage properties of Lithium aluminum deuteride were evaluated using galvanostatic discharge-charge measurements. The potential profiles of the Lithium aluminum deuteride electrode during discharge and charge are shown in Figure 1b and c, respectively. The discharge cycle at a current density of 100 mA/g between 0.005 and 3.0 V vs. Li+/Li reveals that Lithium aluminum deuteride delivers a specific capacity of approximately 1729 mAh/g during the first cycle. This capacity corresponds to approximately 3.5 mol Li. During discharge, the potential drops to a long plateau located at around 0.33 V, followed by a slow decay and a short plateau at approximately 0.14 V. This plateau potential is similar to that of a metallic Al electrode. During charging, a plateau potential is observed at around 0.44 V, along with two sloping potentials. Unfortunately, only about 509 mAh/g (~1.0 mol Li) is recharged in the first charging process, resulting in an initial coulombic efficiency of approximately 29.4%. This indicates that Li3AlH6 after being fully discharged could not be reverted to its initial state completely under the present conditions. In summary, the electrochemical lithium storage properties of Lithium aluminum deuteride reveal a specific capacity of approximately 1729 mAh/g during the first discharge cycle, a plateau potential similar to that of a metallic Al electrode, and a low initial coulombic efficiency. 2
Lithium storage mechanism
The lithium storage mechanism of Lithium aluminum deuteride involves a series of phase transitions and reactions with electrolytes during the discharge and recharge processes. XRD measurements on Lithium aluminum deuteride samples at different lithium storage stages revealed key insights into this mechanism. Initially, Lithium aluminum deuteride is the dominant phase, but as it discharges, side reactions with electrolytes lead to the formation of LiH and metallic Al. As further discharge occurs, new phases like LiAl emerge while Li3AlH6 diminishes. Fully lithiated products consist of LiAl and LiH. The presence of diffraction peaks similar to Al13Fe4 suggests possible Al-Fe intermetallic generation during material preparation. Additionally, unknown phases resulting from Lithium aluminum deuteride-electrolyte reactions were detected, confirmed by FTIR analysis showing characteristic absorption peaks. This detailed characterization highlights the complex structural transformations and interactions underlying the lithium storage mechanism of Lithium aluminum deuteride. 2
Reference
1. Preparation of Lithium Aluminum Hydride. J Am Chem Soc. 1947, 69: 1199.
2. Liang C, Ye Z, Yang Y, et al. Lithium aluminum hydride Li3AlH6: new insight into the anode material for liquid-state lithium-ion batteries. Heliyon. 2023, 9(11): e21765.
);You may like
Related articles And Qustion
Lastest Price from Lithium aluminum deuteride manufacturers
US $39.00-550.00/g2024-02-23
- CAS:
- 14128-54-2
- Min. Order:
- 1g
- Purity:
- 0.97
- Supply Ability:
- 25kg

US $0.00-0.00/g2024-01-08
- CAS:
- 14128-54-2
- Min. Order:
- 10g
- Purity:
- 98%min
- Supply Ability:
- 1 tons