Magnesium carbonate hydroxide: Application and Chemical Studies
General description
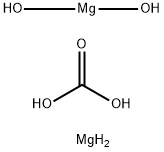
The Magnesium carbonate hydroxide, with the CAS No: 39409-82-0.This chemical’s molecular formula is CH6Mg2O and molecular weight is 146.66. Normally, Magnesium carbonate hydroxide exists as dilute loose powder or crisp monoclinic crystals. It is white, non-toxic, odorless, and stable in air. Slightly soluble in water (solubility 0.02 g per 100 g water at 15 ℃), it is weakly alkaline, and some samples will be decomposed to Mg(OH)2 in high temperature aqueous solution, insoluble in ethanol. It can react with dilute acid and release CO2. When decomposed at 350 ℃, the product is formed including MgO, H2O and CO2. The molecular formula of magnesium carbonate can be summarized as aMgCO3-bMg(OH)2-cH2O, the values of a, b, c vary slightly depending on the preparation method and production process, where a=1 to 4, b=0 to 1, c=0 to 8. [1-4] It is an important inorganic chemical product that is widely used in modern society. Current production rates of magnesium carbonate cannot meet the increasing global demand. This supplydemand imbalance has led many researchers to develop the production process of Magnesium carbonate hydroxide ate.[5]
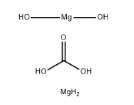
Figure 1 the molecular formula of Magnesium carbonate hydroxide
Applications
Magnesium carbonate hydroxide can be used in pharmaceuticals as an inert vehicle and an adsorbent. Due to its fine texture and high absorbency, it is used in cosmetic manufacturing as a carrier and retainer of perfumes. It is also used in the rubber industry as a reinforcing agent and as an extender for titanium dioxide in paint, lithographing inks and as a precursor for other magnesium-based chemicals. The electronic information industry needs to use functional ceramics, and the preparation process of ceramic components is often used in the Magnesium carbonate hydroxide. Due to its excellent fluidity, Magnesium carbonate hydroxide can be used as a flour anti-caking and loosening agent of flour, increasing the dispersibility and fluidity of flour. It can also supplement the magnesium salt in human body and enhance the heart function. Food grade basic magnesium carbonate is a fine chemical product, the product The requirements are extremely strict, the production scale is small, the output is low, and the added value is high. In addition, food grade Magnesium carbonate hydroxide can also be used as desiccant, color protector, carrier and anti-caking agent, carrier and anti-caking agent, etc.[5]
Preparation
First method: A Mg(OH)2 slurry was obtained by suspending approximately 5.0 g Mg(OH)2 in 125 mL deionised water. The suspension was agitated vigorously with a magnetic stirrer and the pH measured before sparging the CO2. The CO2 was sparged through the solution at 190 mL min 1 while stirring continuously. The final pH of the slurry was obtained by stopping the CO2 flow at the desired pH between 7.5 and 9.0. The slurry was then filtered and the solid product washed with deionised water. The solid product was then dried at the desired temperature. Finally, get Magnesium carbonate hydroxide.[6]
Second mehtod: Dolomite with MgO content of 17% or more is used as raw material. Dolomite and white coal are mixed in the ratio of (8-10):1 and then placed in the kiln at a certain temperature (1000℃). The dolomite clinker is obtained by calcination in the kiln at a certain temperature (1000 to 1200℃). After screening, the clinker is digested in a digestion tank and then made into ash milk. After sieving and digestion in the digestion tank, ash milk is made and further refined to obtain fine ash milk, which is then transferred to Kiln gas from the dolomite calcination stage is purified and then transferred to the carbonate tower. Kiln gas from the dolomite calcination stage is purified and then transferred to the carbonate tower to obtain carbonate. The kiln gas from dolomite calcination stage is purified and then enters the carbonation acid tower for carbonation reaction to get carbonation acid liquid, and the heavy magnesium water clear liquid is obtained by clarification tank; steam heating thermal decomposition After the reaction, through the process of filtration, washing, dehydration, drying, crushing, etc, finally get Magnesium carbonate hydroxide.[7]
Third method: Magnesite carbonation method: The reaction principle of magnesite carbonation is basically the same as that of dolomite carbonation, but because of the high content of magnesium carbonate in magnesite and less impurities, there is no need to recover additional CaCO3. the production process of magnesite carbonation is also largely similar to that of dolomite carbonation, because of the high purity of raw material, the Magnesium carbonate hydroxide produced is of high quality, and the production process is simple and the cost is reduced.[8]
Fourth method: Brine soda method: The raw material of this method is old brine ( containing magnesium chloride, magnesium sulfate and low sodium chloride content) and soda ash. Firstly, after diluting the old brine, add dilute soda ash solution, and then produce magnesium carbonate precipitate after compound decomposition reaction; react until the magnesium ion in the solution is slightly remaining, then stop adding soda ash solution, the product is left to age and then extracted and washed several times to remove the sulfate ion in the solution, and finally the product is made into pulp with water and then pyrolyzed to produce Magnesium carbonate hydroxide.[5]
References
[1] Nashab B. Barringtonite-A new hydrous magnesium carbonate from Barrington Tops, New South Wales, Australia[J]. Mineralogical Magazine, 1965, 34(268): 370- 372.
[2] Liu X W, Feng Y L, LI H R. Preparation of basic magnesium carbonate and its thermal decomposition kinetics in air[J]. Journal of Central South University, 2011, 18: 1865-1870.
[3] HG/T 2959-2010, People's Republic of China Chemical Industry Standard-Industrial hydrated basic magnesium carbonate[S].
[4] Jiang Y P, Peng T J, Sun H J. Preparation of acicular basic magnesium carbonate by the activation product of chrysotile asbestos tailing[J]. Advanced Materials Research, 2010, 178: 230-235.
[5]Zhang G T, Hai C X, Zhou Y. Preparation and Application of Basic Magnesium Carbonate[J]. Journal of salt lank research, 2022, 30(3): 131-138.
[6]Botha A, Strydom C A. Preparation of a magnesium hydroxy carbonate from magnesium hydroxide[J]. Hydrometallurgy, 2001, 62(3):175-183.
[7]Aghiona E, Bronfin B, Eliezer D. The role of the magnesium industry in protecting the environment [J]. Journal of Materials Processing Technology, 2001, 117: 381-385.
[8]Yuan C H,Li H M. Discussion on Methods for the Preparation[J]. Journal of salt lank research, 2005, 13(2): 40-44.
);You may like
Related articles And Qustion
See also
Lastest Price from Magnesium carbonate hydroxide manufacturers

US $1.00/kg2023-09-07
- CAS:
- 39409-82-0
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 50000tons

US $0.00/KG2023-06-29
- CAS:
- 39409-82-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month