Mechanism and Toxicity of Clevudine
Clevudine (L-FMAU) is the unnatural L-enantiomer of the natural nucleoside D-thymidine and has potent antiviral activity against hepadnaviruses including hepatitis B virus (HBV) in vitro and in vivo. Its mode of action is by inhibition of the viral polymerase. Clevudine was discovered at Yale University and licensed to Bukwang, a Korean pharmaceutical company, and also to Pharmasset for further development and marketing. Clevudine is now approved by the Korean Food and Drug Administration (FDA) for the treatment of adults with chronic hepatitis B (CHB), but it is not yet approved by other regulatory agencies. It is only available as tablets for oral administration. The drug is currently undergoing several randomized, double-blind, active control clinical trials in patients with CHB to evaluate its efficacy compared with both lamivudine and adefovir (Clinical Trials Registry Identifiers NCT00362635, NCT00496158, and NCT00496002).
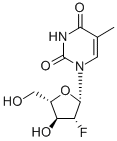
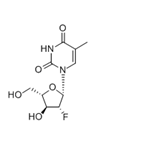
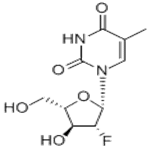
The molecular weight of clevudine is 260. Its chemical name is 2ufluoro- 5-methyl-b-L-arabinofuranosyluracil and the molecular formula is C10H13FN2O5. The chemical structure is shown below.

Uses
Clevudine has been studied as a monotherapy for the treatment of CHB infection.
Mechanism of action
Clevudine is predominantly phosphorylated to the active triphosphate form by cytosolic deoxycytidine kinase in HepG2.2.15 cells and HepG2 cells, and, although both thymidine kinase and mitochondrial deoxypyrimidine kinase can also phosphorylate clevudine, their significance is uncertain. In human hepatocytes treated with an equivalent dose of clevudine that approximates the human plasma Cmax for a 30-mg dose, clevudine is efficiently phosphorylated and achieves an intracellular concentration of clevudine triphosphate that is 300- to 500-fold higher than the inhibition constant for the HBV polymerase (Ki 0.12 mM) .
Clevudine preferentially inhibits DNA-dependent DNA polymerase activity but may also inhibit reverse transcription, and therefore minus-strand HBV DNA synthesis. Clevudine triphosphate acts as a competitive inhibitor by binding to the catalytic center of the HBV polymerase, thereby preventing the addition of natural nucleoside substrates into the elongating DNA chain. However, the unique conformation of clevudine triphosphate prevents the addition of clevudine triphosphate into the nascent viral DNA and consequently the drug does not act as a chain terminator.
Pharmacokinetics and Pharmacodynamics
Pharmacokinetic variables were determined in a phase II doseescalation trial in patients with CHB. Patients were treated for 28 days with clevudine at doses between 10 and 200 mg daily. Pharmacokinetic evaluation was not possible in the 10 mg dose cohort because serum levels were too low to permit reliable analyses. Steady state serum concentrations were achieved after 22 days of treatment, with a mean half-life ranging from 44 to 60 hours.
The bioavailability of clevudine is approximately 60% in rats and between 20% and 40% in woodchucks. Up to 15% of clevudine is bound to plasma proteins and the drug is predominantly eliminated in the urine unchanged. Steady-state volume of distribution in rats and woodchucks is greater than total volume of body water, indicating that clevudine is predominantly distributed intracellularly.
Toxicity
Cytotoxicity of clevudine has been tested in vitro in HepG 2.2.15, MT2, CEM, H1, and bone marrow progenitor cells. In all cells tested, the 50% cytotoxic concentrations exceed 100 mM. Furthermore, mitochondrial toxicity was not seen. At concentrations of up to 100 mM, mitochondrial DNA synthesis and serum lactic acid levels are not affected. Clevudine triphosphate is neither a substrate nor an inhibitor for human DNA polymases a, X, g, and d, and radiolabeled clevudine is not incorporated into cellular DNA.
Commonly reported adverse events include infection, asthenia, dyspepsia, abdominal pain, and headache. Elevations in alanine aminotransferase (ALT) were more frequently seen in the placebo group than in the clevudine group.
);You may like
See also
Lastest Price from Clevudine manufacturers
US $0.00-0.00/kg2022-09-24
- CAS:
- 163252-36-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton
US $0.00/KG2022-02-10
- CAS:
- 163252-36-6
- Min. Order:
- 1KG
- Purity:
- 98.9%
- Supply Ability:
- 100 tons