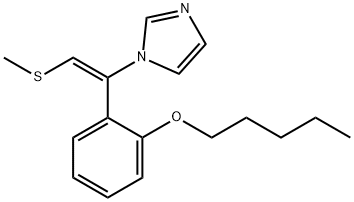
Mechanism of action of Neticonazole
Neticonazole is a vinyl imidazole similar to lanoconazole with the chemical name [E)-1-(2-methylthio)-1-[o-(pentyloxy)phenyl]vinyl]imidazole hydrochloride; the chemical structure is shown below. It has been developed as a topical formulation and marketed in Japan since 1993, where it is indicated for treatment of dermatomycoses. It is one of the most widely used imidazoles in Japan.

Mechanism of action
The primary mechanism of action of neticonazole is similar to that of other imidazoles, that is, inhibition dependent C-14ademethylation of lanosterol, preventing conversion to ergosterol. The resulting depletion of normal fungal sterols (ergosterol) and accumulation of 14a-methyl sterols (lanosterol) in the fungal cell membrane induces damage to the cell. Tatsumi et al.established that neticonazole concentrations of 0.25 mg/ml were fungicidal for T. mentagrophytes and T. rubrum.
Uses
In an open-label study, neticonazole 1% cream alone was compared with neticonazole 1% cream plus an occlusive dressing in the treatment of 96 patients with chronic hyperkeratotic tinea pedis. Following 4 weeks of therapy, clinical cure was observed in 75% of patients treated with neticonazole alone versus 52% of patients treated with neticonazole plus dressing.
Dosage
Neticonazole is available as a topical formulation of 1% neticonazole hydrochloride cream and a 1% solution.
Toxicity
Allergic contact dermatitis after topical application of neticonazole in humans has been reported in at least 10 cases from Japan. Cross-sensitization was observed between lanoconazole and neticonazole ointments.
);