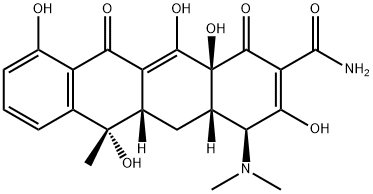
Mechanism of action of Tetracycline
Tetracycline was first described in 1953 and was prepared from chlortetracycline at Lederle Laboratories, and was also independently derived from oxytetracycline at Pfizer Laboratories. Tetracycline is a bacteriostatic antibiotic which shares a four benzene ring structure with the other tetracyclines.
It is only available for oral administration and has broad-spectrum activity against bacteria, including cell wall-deficient organisms and parasites, such as Plasmodia. Other tetracyclines, doxycycline and minocycline, are used much more widely in clinical practice because of their increased bioavailability and lipophilicity, which increases their spectrum and degree of activity. Tetracycline acts on ribosomal targets to reduce protein synthesis. Introduced soon after penicillin G and the sulfonamides, the tetracyclines were once widely used.
MECHANISM OF DRUG ACTION
Tetracyclines inhibit bacterial protein synthesis. They bind principally to the 30S subunits of bacterial ribosomes and specifically inhibit the enzyme binding of aminoacyl-tRNA to the adjacent ribosomal acceptor site, blocking the elongation phase. The binding of tetracyclines to the target is reversible, possibly explaining their bacteriostatic properties. They may also cause alterations in the cytoplasmic membrane, thereby allowing leakage of nucleotides and other compounds from the cell. This action would explain the rapid inhibition of DNA replication that ensues when cells are exposed to concentrations of tetracycline in excess of that needed for protein inhibition. In addition, tetracyclines appear to inhibit adhesion of bacteria to human cells, reducing bacterial pathogenicity. Tetracycline probably inhibits the synthesis of a specific protein in the bacterial cell surface. In higher concentrations, tetracyclines also inhibit mammalian protein synthesis. This antianabolic effect is of clinical significance because it may aggravate pre-existing renal functional impairment. In addition, these drugs may interfere with parenteral nutrition in postoperative patients by inhibiting the utilization of amino acids for protein synthesis.
Demethylchlortetracycline or demeclocycline was obtained from a mutant of Duggar’s original strain of Streptomyces aureofaciens and reported in 1957. Its main current use is for the treatment of the rare patient with the syndrome of inappropriate antidiuretic hormone secretion who is unresponsive to other measures. The major side-effect of this agent is nephrogenic diabetes insipidus. Tetracycline compounds are mainly marketed for oral administration, but preparations of doxycycline and minocycline for i.v. administration are available. Two other compounds have been available specifically for parenteral use: rolitetracycline (pyrrolidino-methyl-tetracycline) and rolitetracycline nitrate (pyrrolidino-methyltetracycline nitrate).
);You may like
See also
Lastest Price from Tetracycline manufacturers
US $0.00-0.00/kg2023-11-01
- CAS:
- 60-54-8
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 99.0%min
- Supply Ability:
- 200kg

US $0.00/KG2023-08-30
- CAS:
- 60-54-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month