Methanol:General description,Application,and Production
Methanol, also known as methyl alcohol, wood alcohol, or carbinol, is a clear, colorless liquid with a slightly sweet odor. It is the simplest type of alcohol and has the chemical formula CH3OH. Methanol is highly flammable and toxic, and it should be handled with care. It is the primary alcohol that is the simplest aliphatic alcohol, comprising a methyl and an alcohol group. It has a role as an amphiprotic solvent, a fuel, a human metabolite, an Escherichia coli metabolite, a mouse metabolite and a Mycoplasma genitalium metabolite. It is an alkyl alcohol, a one-carbon compound, a volatile organic compound and a primary alcohol. It is a conjugate acid of a methoxide.
Application and Pharmacology
Methanol is used in a variety of industrial applications, such as in the production of formaldehyde, acetic acid, and other chemicals. It is also used as a solvent, fuel, and antifreeze. Methanol is often used as a fuel for racing cars, and it is also used as a fuel additive to increase octane levels in gasoline. The methanol produced can further be utilized as a clean-burning fuel, in pharmaceuticals, automobiles and as a general solvent in various industries etc.
1. Synthetic methylotrophy is the development of non-nativemethylotrophs that can utilize methane and methanol as sole carbon and energy sources or as co-substrates with carbohydrates to produce metabolites as biofuels and chemicals. The availability of methane (from natural gas) and its oxidation product, methanol, has been increasing, while prices have been decreasing, thus rendering them as attractive fermentation substrates. As they are more reduced than most carbohydrates, methane and methanol, as co-substrates, can enhance the yields of biologically produced metabolites. Here we discuss synthetic biology and metabolic engineering strategies based on the native biology of aerobic methylotrophs for developing synthetic strains grown on methanol, with Escherichia coli as the prototype.
2. In pharmacology, methanol is used as an antidote for ethylene glycol and methanol poisoning. Methanol is converted into formaldehyde and then into formic acid in the body. Formic acid is responsible for the toxic effects of methanol and can cause metabolic acidosis, blindness, and even death. The administration of ethanol or fomepizole can inhibit the formation of formic acid, thus preventing the toxic effects of methanol.
3. Methanol has also been investigated as a potential alternative fuel for internal combustion engines. Methanol can be produced from renewable sources, such as biomass, and it has a high octane rating, which can improve engine performance. Methanol is also relatively low in emissions, which can help reduce air pollution. In recent years, there has been growing interest in methanol as a potential alternative fuel for internal combustion engines. Methanol is a renewable fuel that can be produced from a variety of sources, including natural gas, coal, and biomass. One study published in the Journal of Energy and Power Engineering in 2014 investigated the combustion characteristics of methanol in a spark-ignition engine. The study found that methanol combustion produced lower emissions of carbon monoxide, hydrocarbons, and nitrogen oxides compared to gasoline combustion. The study also found that methanol combustion produced higher thermal efficiency and indicated mean effective pressure (IMEP) compared to gasoline combustion. Overall, these studies suggest that methanol has potential as a renewable and low-emission alternative fuel for internal combustion engines. However, further research is needed to fully understand the potential benefits and challenges of using methanol as a fuel[1].
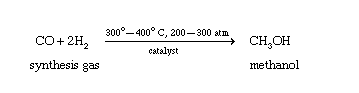
Synthesis
Methanol is produced from natural gas or coal, and it can also be produced by the fermentation of biomass. However, it is important to note that methanol is a toxic substance and should not be ingested or inhaled. Exposure to methanol can cause serious health problems, such as blindness, liver damage, and even death[2,3].
1. Materials such as MOFs, porphyrins, and nanomaterials are also used widely for CO2 absorption and conversion into methanol. Thus, a combination of enzymes and materials which convert the CO2 into methanol could energize the CO2 utilization. The CO2 to methanol conversion utilizes carbon better than the conventional syngas and the reaction yields fewer by-products. The methanol produced can further be utilized as a clean-burning fuel, in pharmaceuticals, automobiles and as a general solvent in various industries etc. This makes methanol an ideal fuel in comparison to the conventional petroleum-based ones and it is advantageous for a safer and cleaner environment. In this review article, various aspects of the circular economy with the present scenario of environmental crisis will also be considered for large-scale sustainable biorefinery of methanol production from atmospheric CO2.
2. The methanol formation from direct CO2 hydrogenation is deemed to be a green approach for the synthesis of sustainable fuels. For the hydrogenation of CO2 into methanol two different mechanisms have been proposed: (a) the formate pathway where the hydrogenation of CO2 produces formate species and (b) hydrocarboxyl pathway in which hydrogenation of CO2 to methanol via formyl and formate intermediates. Also, formate species were the most common intermediate in both pathways (Figure. 1). Over the last decade, several efforts have been made for developing an effective catalyst for the hydrogenation of CO2 to methanol. The various research has shown that Pd nanoparticles supported on metal oxides have excellent activities for hydrogenation of CO2 to methanol even under low-temperature conditions. In a previous study, the CO2 hydrogenation activity of bi-metallic Pd–Cu catalysts was compared with other bimetallic catalysts. These intermetallic nanoparticles were observed to be most efficient as they showed 70% higher methanol rates during liquid phase methanol synthesis experiments, compared to the previously used heterogeneous catalyst, Cu/ZnO/Al2O3 which showed only 45% methanol selectivity. Cu/ZnO/Al2O3 showed low methanol selectivity, stability, and average catalytic activity[4].

Figure 1 Mechanism of methanol synthesis by CO2 hydrogenation through hydrocarboxyl and formate pathway.
3. Improvement in methanol production by regulating the composition of synthetic gas mixture and raw biogas:Raw biogas can be an alternative feedstock to pure methane (CH4) for methanol production. In this investigation, we evaluated the methanol production potential of Methylosinus sporium from raw biogas originated from an anaerobic digester. Furthermore, the roles of different gases in methanol production were investigated using synthetic gas mixtures of CH4, carbon dioxide (CO2), and hydrogen (H2). Maximum methanol production was 5.13, 4.35, 6.28, 7.16, 0.38, and 0.36 mM from raw biogas, CH4:CO2, CH4:H2, CH4:CO2:H2, CO2, and CO2:H2, respectively. Supplementation of H2 into raw biogas increased methanol production up to 3.5-fold. Additionally, covalent immobilization of M. Sporium on chitosan resulted in higher methanol production from raw biogas.
Toxicity and safety
Methanol is a highly toxic substance and can pose serious health risks if ingested or inhaled. The primary route of exposure to methanol is through inhalation of its vapor or ingestion of methanol-containing products. Methanol can also be absorbed through the skin, but this route of exposure is less common. Ingestion of methanol can cause a range of symptoms, including headache, dizziness, nausea, vomiting, abdominal pain, and loss of consciousness. Methanol can also cause severe damage to the central nervous system, liver, and kidneys, and can lead to blindness or even death. Inhalation of methanol vapor can cause irritation to the respiratory tract, eyes, and skin. Prolonged exposure to methanol vapor can cause headache, dizziness, nausea, and vomiting. Chronic exposure to methanol vapor can cause liver and kidney damage, and may also lead to neurological problems. It is important to handle methanol with care and to follow proper safety procedures to minimize the risk of exposure. Protective equipment such as gloves, goggles, and respiratory protection should be worn when handling methanol. Methanol should also be stored in a cool, dry, and well-ventilated area away from sources of heat and ignition. In the event of exposure to methanol, immediate medical attention should be sought. Treatment may involve administration of antidotes, supportive care, and monitoring of organ function.
Reference
1. Reed T. B. & Lerner R. M., "Methanol: A Versatile Fuel for Immediate Use: Methanol can be made from gas, coal, or wood. It is stored and used in existing equipment," Science, Vol.182, No.4119(1973), pp.1299-1304.
2.
3. Whitaker W. B., Sandoval N. R. & Bennett R. K. et al., "Synthetic methylotrophy: engineering the production of biofuels and chemicals based on the biology of aerobic methanol utilization," Curr Opin Biotechnol, Vol.33(2015), pp.165-175.
4.
);You may like
Related articles And Qustion
Lastest Price from Methanol manufacturers

US $10.00/kg2024-04-26
- CAS:
- 67-56-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 tons

US $1.00/g2024-04-25
- CAS:
- 67-56-1
- Min. Order:
- 1g
- Purity:
- 99
- Supply Ability:
- 20tons