N-Glycyl-L-tyrosine: Overview, Applications in Synthesis of Triorganotin Derivatives and Preparation
General Description
N-Glycyl-L-tyrosine is a peptide derivative composed of glycine and tyrosine, showing potential in biological and pharmacological applications. It may function as a signaling molecule, interact with biomolecules, and serve as a building block for larger peptides or proteins. In pharmacology, it holds promise as a potential therapeutic agent. Furthermore, studies have demonstrated its role in the synthesis of triorganotin derivatives with anti-inflammatory, antimicrobial, and cardiovascular activities. The compound can be prepared through a cost-effective and environmentally friendly method, offering high yield and simplicity. Overall, N-Glycyl-L-tyrosine's unique properties make it a compelling candidate for various biomedical applications and pharmaceutical development.

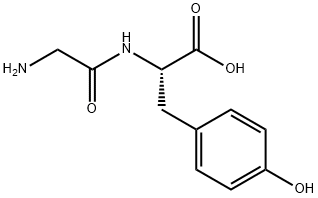
Figure 1. N-Glycyl-L-tyrosine
Overview
N-Glycyl-L-tyrosine, a peptide derivative, is a biochemical compound that holds significant importance in the realm of biochemistry and pharmacology. This molecule is composed of glycine and tyrosine amino acids, linked via a peptide bond. Glycine, the smallest amino acid, possesses a nonpolar side chain, conferring unique physicochemical properties to the peptide. Tyrosine, on the other hand, is an aromatic amino acid with a hydroxyl group on its side chain, contributing to the compound's biological activity. The presence of these amino acids in N-Glycyl-L-tyrosine suggests its potential involvement in various biological processes. It may act as a signaling molecule, regulating cellular functions or metabolic pathways. Additionally, its structural features may enable it to interact with other biomolecules, such as enzymes or receptors, modulating their activity. Moreover, N-Glycyl-L-tyrosine could serve as a building block for larger peptides or proteins, playing a crucial role in protein synthesis or post-translational modifications. Its specific sequence and structure could determine its unique function within biological systems. In pharmacological applications, N-Glycyl-L-tyrosine could be explored as a potential therapeutic agent. Its ability to interact with specific targets within the body could lead to the development of novel drugs for treating various diseases. However, further research is necessary to fully understand its biological effects and potential therapeutic uses. In summary, N-Glycyl-L-tyrosine is a peptide derivative with unique structural and biochemical properties that could hold promise in various biological and pharmacological applications. Its role in biological processes and potential therapeutic uses remain an exciting area of research. 1
Applications in synthesis of triorganotin derivatives
N-Glycyl-L-tyrosine, represented as H2L-5 in a study, plays a significant role in the synthesis of new triorganotin(IV) derivatives of dipeptides with potential biomedical applications. These derivatives, such as R3Sn(HL), have been investigated for their anti-inflammatory, antimicrobial, and cardiovascular activities. The compounds were synthesized and characterized using various spectroscopic techniques. In vivo studies utilizing a carrageenan-induced paw edema bioassay in rats revealed that the triphenyltin(IV) derivatives exhibited anti-inflammatory activity comparable to phenylbutazone, a common anti-inflammatory drug, with a high safety margin. Additionally, Ph3Sn(Gly-Val) demonstrated potent cardiovascular activity. Furthermore, the compounds displayed appreciable antibacterial activities against a range of bacteria, including Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Candida albicans, Microsporum gypseum, and Euglena gracilis. Notably, Ph3Sn(Gly-Gly) and Ph3Sn(Gly-Val) emerged as the most distinctive derivatives due to their promising in vivo anti-inflammatory activity and in vitro antibacterial activity against both gram-positive and gram-negative bacteria. These findings suggest the potential of N-Glycyl-L-tyrosine-based derivatives as candidates for the development of new anti-inflammatory, antimicrobial, and cardiovascular agents. 2
Preparation
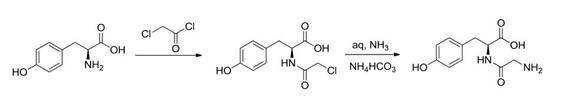
The process for the preparation of N-Glycyl-L-tyrosine involves a series of steps that result in a high-yield, cost-effective, and environmentally friendly method. Firstly, the process begins by preparing glycyl chloride hydrochloride from glycine and thionyl chloride. This step is crucial as it sets the foundation for the subsequent synthesis. Thionyl chloride reacts with glycine to form glycyl chloride hydrochloride, an important intermediate in the production of N-Glycyl-L-tyrosine. Following this, the next step involves the preparation of N-Glycyl-L-tyrosine from the glycyl chloride hydrochloride and L-tyrosine under de-acidifying agent in a solution containing water. The use of a de-acidifying agent helps to facilitate the reaction and ensure the formation of the desired product. It is noteworthy that this method offers several distinct advantages, including high yield, simplicity in operation, low cost, and minimal environmental pollution. By employing this process, researchers and manufacturers can efficiently obtain N-Glycyl-L-tyrosine, an important compound with various applications in pharmaceuticals, biochemistry, and related fields. Additionally, the simplicity and cost-effectiveness of this method make it an attractive option for large-scale production, offering potential benefits for industries and research endeavors seeking to utilize N-Glycyl-L-tyrosine in their products or studies. 3
Reference
1. Glycyl-L-tyrosine. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 92829.
2. Nath M, Pokharia S, Eng G, Song X, Kumar A. New triorganotin(IV) derivatives of dipeptides as anti-inflammatory-antimicrobial agents. Eur J Med Chem. 2005; 40(3): 289-298.
3. Yang H, Zhang T, Zhang X. Process for preparation of glycyl tyrosine. 2013; Patent Number: CN103172695.
);You may like
Related articles And Qustion
Lastest Price from N-Glycyl-L-tyrosine manufacturers
US $0.00-0.00/kg2024-04-30
- CAS:
- 658-79-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1T+
US $0.00-0.00/kg2024-04-26
- CAS:
- 658-79-7
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 99%
- Supply Ability:
- 20tons