N,N-Dimethylbenzylamine: applications and safety
General Description
N,N-Dimethylbenzylamine finds extensive application in the production of epoxy resins, which are widely used in industries such as electronics, aerospace, automotive, and construction. It acts as a curing agent, promoting the cross-linking reaction and resulting in high strength, chemical resistance, and thermal stability of epoxy resins. It also enhances adhesion properties, enabling strong bonds with various substrates. Additionally, N,N-Dimethylbenzylamine serves as an efficient organocatalyst in the synthesis of dihydropyrano[3,2-c]chromene derivatives, offering mild reaction conditions, reusability of the reaction medium, shorter reaction times, and easy separation of the reaction mixture. However, it poses risks to human health and the environment, requiring proper safety precautions. It can cause severe skin burns, eye damage, and is toxic to aquatic life. Measures such as protective gear and immediate medical attention are necessary when handling this compound.

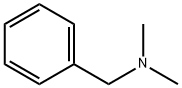
Figure 1. N,N-Dimethylbenzylamine
Applications
Production of epoxy resins
N,N-Dimethylbenzylamine is an organic compound that finds extensive application in the production of epoxy resins. Epoxy resins are widely used in various industries, such as electronics, aerospace, automotive, and construction, due to their excellent adhesive and mechanical properties. N,N-Dimethylbenzylamine acts as an effective curing agent in epoxy resin production. It plays a crucial role in the curing process by promoting the cross-linking reaction between epoxy monomers and hardeners, resulting in the formation of a three-dimensional network structure. This network imparts the desirable properties to epoxy resins, such as high strength, chemical resistance, and thermal stability. One of the key advantages of using N,N-Dimethylbenzylamine is its ability to accelerate the curing process, significantly reducing the processing time required for epoxy resin production. Moreover, DMBA offers flexibility in controlling the reactivity and pot life of epoxy systems, allowing manufacturers to adjust the formulation according to specific application requirements. In addition, N,N-Dimethylbenzylamine enhances the adhesion properties of epoxy resins, enabling strong bonds with various substrates, including metals, plastics, and composites. This feature is particularly advantageous in industries where bonding strength and durability are essential, such as in structural adhesives and coatings. Overall, the incorporation of N,N-Dimethylbenzylamine in the production of epoxy resins enhances their performance characteristics, making them indispensable in numerous industrial applications. 1
Organocatalyst
N,N-Dimethylbenzylamine is a widely available organocatalyst that has found significant application in the field of organic synthesis. One of its notable applications is as a catalyst for the one-pot synthesis of dihydropyrano[3,2-c]chromene derivatives in an ethanol medium. In a study, a novel one-pot Knoevenagel-Michael-Thorpe-Ziegler type cascade heterocyclization reaction was developed using N,N-Dimethylbenzylamine as the catalyst. The reaction involved a multicomponent condensation reaction of 4-hydroxycoumarin, malononitrile/ethyl cyanoacetate, and various aldehydes at a temperature of 60°C. The use of N,N-Dimethylbenzylamine as a catalyst in this reaction offers several advantages. Firstly, the reaction conditions are relatively mild, ensuring high product yields and minimizing unwanted side reactions. Secondly, the ethanol medium used in the reaction is reusable, providing cost-effectiveness and reducing waste generation. Additionally, the reaction times are shorter, allowing for increased efficiency in production. Lastly, the separation of the reaction mixture is straightforward, facilitating the purification process. In summary, N,N-Dimethylbenzylamine serves as an efficient and commercially available catalyst for the one-pot synthesis of dihydropyrano[3,2-c]chromene derivatives. Its application in this cascade heterocyclization reaction enables mild reaction conditions, reusability of the reaction medium, shorter reaction times, and easy separation of the reaction mixture. 2
Safety
N,N-Dimethylbenzylamine poses potential risks to human health and the environment. DMBA is harmful upon contact with the skin, if swallowed, and if inhaled. It can cause severe skin burns and eye damage. Additionally, it has the potential to be toxic to aquatic life with long-lasting effects. Moreover, DMBA is classified as a flammable liquid and vapor, which presents fire hazards. To ensure safety when working with DMBA, it is important to follow the recommended precautions specified. These include wearing protective gloves, clothing, eye protection, and face protection. In case of exposure or ingestion, immediate medical attention should be sought by calling a poison center or consulting a doctor/physician. If DMBA comes into contact with the eyes, they should be rinsed cautiously with water for several minutes, and contact lenses should be removed if present. In summary, N,N-Dimethylbenzylamine is associated with various potential hazards, including harm to humans upon contact, ingestion, or inhalation, as well as the risk of causing severe burns and eye damage. It is also harmful to aquatic life. Therefore, appropriate precautionary measures must be taken to ensure safe handling and minimize the associated risks. 3
Reference
1. St?hlbom B, Akesson B. N,N-dimethylbenzylamine: occupational exposure, analysis and biological monitoring. Int Arch Occup Environ Health. 1995;67(3):159-164.
2. Kiyani H, Jalali MS. Facial and Efficient Access to Dihydropyrano[3,2-c]Chromenes via Three-Component Reaction Using N,N-Dimethylbenzylamine As a New Organocatalyst. Comb Chem High Throughput Screen. 2016;19(4):275-282.
3. SAFETY DATA SHEET: N,N-Dimethylbenzylamine. Thermo Fisher SCIENTIFIC, 2000, Cat No. : AC159750000.
);You may like
Related articles And Qustion
See also
Lastest Price from N,N-Dimethylbenzylamine manufacturers

US $0.00/KG2023-06-30
- CAS:
- 103-83-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month

US $10.60/KG2023-03-06
- CAS:
- 103-83-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5000kg

![3680-69-1 Properties of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidineapplications of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidinesafety of 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/NewsImg/2023-09-25/6383126719184002941108282.jpg)