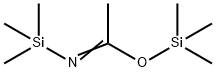
N, O-Bis(trimethylsilyl)acetamide-a widely used Silylation Reagents
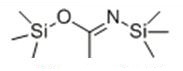
N, O-bis(trimethylsilyl)acetamide (BSA) is neutral silane protective agent, mainly used for the protection of amino acids, carboxylic acids, alcohols and amides. Meanwhile, BSA is used in passivation of chromatographic carriers and modification of polar groups of samples. However, interference derived from excess BSA was unavoidable in the analysis. A perfect substitute of BAS, N, O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) was introduced. BSTFA is one of the most widely used silylation reagents due to the volatility of itself and its byproducts, less tendency to have interference with the trimethylsilyl derivatives of the analytes and non-corrosive byproducts. Based on the advantages of BSTFA, it was chosen to derivatize 1C2P and 2C1P in the food additive extracts to minimize the matrix effects and peak co-elution issues.
Also, BSA is an important pharmaceutical intermediate, mainly used in the production of synthetic cephalosporin antibiotics, which can improve the yield and reduce the toxicity of the product. The coupling of 6-halouracil bases to ribose was found to be problematic but an efficient strategy combining BSA as a silylating agent and TMSOTf as a Lewis acid catalyst was successfully employed. The coupling method described here should be of interest in the synthesis of nucleoside derivatives involving sterically hindered pyrimidine or purine bases. A method using N, O-bis(trimethylsilyl)acetamide/N-hydroxysuccinimide ester (BSA/NHS) as coupling agents for dipeptide synthesis is descried. The coupling reaction between NHS and amines could be performed under mild conditions with BSA as coupling reagent and no additional acid/base is required. All byproducts and excessive reactants are water soluble or hydrolysable and easy to eliminate through water-washing at the purification stage. Moreover, all the reactants are inexpensive and widely used in conventional drug production.
Using BSA as catalysts, in combination with a series of ionic liquids with fluoride anions, the α, β-unsaturated esters synthesis. The mechanism investigation through GC-MS indicates that BSA would convert into onium amide, which acted as a strong base for α-H abstraction. By the way, BSA is also used in the removal of water from an anhydrous reaction system.
References
[1] Zhu, K.; Gu, B.; Kerry, M.; et al. Elimination of N, O-bis(trimethylsilyl)trifluoroacetamide interference by base treatment in derivatization gas chromatography mass spectrometry determination of parts per billion of alcohols in a food additive, J. Chromatography A, 2017, 1490(24): 74-79.
[2] Blackburn, D. J.; Kent, G. T.; Wu, W. Regiospecific synthesis of 6-halouridine derivatives: An effective method for coupling sterically hindered pyrimidine bases to ribose, Tetrahedron Lett., 2017, 58(13): 1348-1350.
[3] Huang, Y.; Feng, W.-H. N, O-Bis(trimethylsilyl)acetamide/N-hydroxysuccinimide ester (BSA/NHS) as coupling agents for dipeptide synthesis, Chinese Chem. Lett., 2016, 27(3): 357-360.
[4] Wang, G.; Xu, Y.; Zhang, S.; et al. An ionic liquid catalyzed probase method for one-pot synthesis of α, β-unsaturated esters from esters and aldehydes under mild conditions, Green Chem., 2017, 20
You may like
See also
Lastest Price from N,O-Bis(trimethylsilyl)acetamide manufacturers
US $8.00-0.80/KG2024-03-25
- CAS:
- 10416-59-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $60.00/KG2024-02-27
- CAS:
- 10416-59-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 tons