N-Phenyl-bis(trifluoromethanesulfonimide): Pharmaceutical Synthesis Applications and Green Preparation
General Description
N-Phenyl-bis(trifluoromethanesulfonimide) is a versatile compound used in the synthesis of antibacterial agents, such as benzothiazole derivatives, with potent antibacterial properties. It also plays a crucial role in creating heterocyclic compounds that inhibit LRRK2 kinase activity, showing promise in treating diseases like Parkinson's and Alzheimer's. Additionally, a green synthesis method for N-Phenyl-bis(trifluoromethanesulfonimide) offers an environmentally friendly approach to producing this compound efficiently. Overall, these applications highlight the importance of N-Phenyl-bis(trifluoromethanesulfonimide) in developing novel pharmaceuticals with significant therapeutic potential.

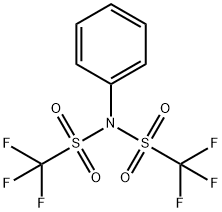
Figure 1. N-Phenyl-bis(trifluoromethanesulfonimide)
Pharmaceutical Synthesis Applications
Antibacterial agents
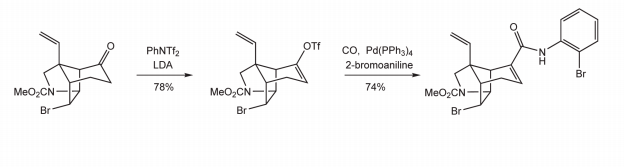
N-Phenyl-bis(trifluoromethanesulfonimide) serves as a crucial reactant in the preparation of antibacterial agents, particularly benzothiazole derivatives. These derivatives, exemplified by compound II, are instrumental in combating various bacterial infections. In the synthesis process, N-Phenyl-bis(trifluoromethanesulfonimide) participates in multi-step reactions, contributing to the formation of the desired benzothiazole derivatives (compound II) as outlined in the provided chemical structure. This compound, along with its analogs, exhibits potent antibacterial properties, with minimum inhibitory concentrations (MIC) below 0.25 μg/mL against a spectrum of bacterial strains, including chlamydophila pneumoniae, enterococcus faecalis, and staphylococcus aureus. Moreover, the versatility of N-Phenyl-bis(trifluoromethanesulfonimide) enables the synthesis of diverse benzothiazole derivatives, tailored to enhance antibacterial efficacy and target specific bacterial strains. These findings underscore the significance of N-Phenyl-bis(trifluoromethanesulfonimide) as a key reagent in the development of novel antibacterial agents, addressing the pressing need for effective treatments against microbial infections. 1
Heterocyclic compounds with inhibiting LRRK2 kinase activity
N-Phenyl-bis(trifluoromethanesulfonimide) serves as a crucial reactant in the synthesis of heterocyclic compounds designed to inhibit LRRK2 kinase activity. These heterocyclic compounds encompass a diverse array of structures tailored to interact with the LRRK2 kinase enzyme, thereby modulating its activity. The structural diversity within these compounds allows for precise tuning of pharmacological properties, enhancing their efficacy and selectivity as kinase inhibitors. The preparation of these heterocyclic compounds involves a multistep synthetic approach, utilizing N-Phenyl-bis(trifluoromethanesulfonimide) as a key building block. Various substituents are strategically introduced to the heterocyclic scaffold to optimize the compound's potency and pharmacokinetic profile. These novel compounds exhibit promising inhibitory effects against LRRK2 kinase activity, as demonstrated through in vitro assays. Moreover, they hold potential therapeutic value in the treatment or prevention of diseases associated with aberrant LRRK2 kinase activity, such as Parkinson's disease, Alzheimer's disease, and amyotrophic lateral sclerosis (ALS). One specific compound, 4-(6-(6-(3,3-Difluoro-1-methylpiperidin-4-yl)-5-methyl-1H-indazol-1-yl)-2-methoxypyrimidin-4-yl)morpholine enantiomer (II), was synthesized and evaluated for its inhibitory potency against LRRK2 kinase activity, yielding promising results in both recombinant cellular and human LRRK2 inhibition assays. These findings underscore the potential therapeutic relevance of N-Phenyl-bis(trifluoromethanesulfonimide)-derived heterocyclic compounds in combating diseases associated with dysregulated LRRK2 kinase activity. 2
Green Preparation
The green preparation of N-Phenyl-bis(trifluoromethanesulfonimide) offers a sustainable and efficient method for synthesizing this compound. The process involves the following steps: Firstly, trifluoromethanesulfonic acid and an organic base, such as N,N-diisopropylethyl acetate amine, are dissolved in an organic solvent, such as dichloromethane. This mixture is then combined with HATU (2-(7-azabenzotriazole)-N,N,N',N'-tetramethylurea hexafluorophosphate) at a controlled temperature (≥15°C) to form a reaction solution containing trifluoromethanesulfonic acid active ester. It's crucial to maintain the reaction temperature to ensure that the organic solvent doesn't boil. Next, aniline is added to the reaction solution, and the mixture is allowed to react at a specific temperature (25°C ± 5°C) for 6 to 12 hours. After the reaction is complete, the organic solvent is removed to obtain a crude product containing N-Phenyl-bis(trifluoromethanesulfonimide). The crude product is then washed and subjected to purification and recrystallization using an alcohol solvent with 1 to 3 carbon atoms. This results in the isolation of N-Phenyl-bis(trifluoromethanesulfonimide) in high purity. This method offers several advantages, including high purity and yield of the desired compound, mild reaction conditions, high utilization rate of raw materials, and environmental friendliness. By employing this green synthesis route, N-Phenyl-bis(trifluoromethanesulfonimide) can be efficiently produced with minimal impact on the environment. 3
Reference
1. Haydon DJ. Czaplewski LG, Palmer NJ. Preparation of benzothiazole derivatives as antibacterial agents. 2012; Patent Number: US20120004221.
2. Ding X, Jin Y, Liu Q. Preparation of heterocyclic compounds that inhibit LRRK2 kinase activity. 2017; Patent Number: WO2017012576.
3. Hua YX, Wang W, Ma LJ. Green preparation of N-phenylbis(trifluoromethanesulfonyl)imide. 2022; Patent Number: CN113880733.
);You may like
Related articles And Qustion
See also
Lastest Price from N-Phenyl-bis(trifluoromethanesulfonimide) manufacturers

US $0.00-0.00/KG2024-02-01
- CAS:
- 37595-74-7
- Min. Order:
- 10mg
- Purity:
- 99%HPLC
- Supply Ability:
- 2000tons

US $65.00-650.00/kg2024-01-02
- CAS:
- 37595-74-7
- Min. Order:
- 10kg
- Purity:
- 0.99
- Supply Ability:
- 20tons