New Drug Application to FDA for UX007 (triheptanoin)
NOVATO, Calif., Aug 01, 2019 (GLOBE NEWSWIRE via COMTEX) -- NOVATO, Calif., Aug. 01, 2019 (GLOBE NEWSWIRE) -- Ultragenyx Pharmaceutical Inc. RARE, +1.66%, a biopharmaceutical company focused on the development of novel products for serious rare and ultra-rare diseases, today announced that it has submitted to the U.S. Food and Drug Administration (FDA) a New Drug Application (NDA) for UX007 (triheptanoin) for the treatment of long-chain fatty acid oxidation disorders (LC-FAOD), a group of genetic disorders in which the body is unable to convert long-chain fatty acids into energy. The FDA previously granted Rare Pediatric Disease Designation and Fast Track designation, which enables eligibility for Priority Review, if relevant criteria are met. Ultragenyx expects to hear back from FDA on submission acceptance and review designation within 60 days.
"Many patients with long-chain fatty acid oxidation disorders have difficult lives with frequent hospitalizations despite the best current care, and we believe our data suggest that treatment with UX007 can reduce these major medical events over a sustained period of time," said Camille L. Bedrosian, M.D., Chief Medical Officer of Ultragenyx. "The submission of this NDA is an important step toward providing a new treatment option and we look forward to working with the FDA on this review."
The NDA submission is supported by a comprehensive package of data including results from a company-sponsored Phase 2 study of UX007 in 29 patients, a long-term safety and efficacy extension study in 75 patients including 20 patients who were previously naive to UX007, a retrospective medical record review of 20 original compassionate use patients, 67 patients treated through expanded access, and a randomized controlled investigator-sponsored study of 32 patients showing an effect on cardiac function.
About LC-FAOD
LC-FAOD are a group of autosomal recessive genetic disorders characterized by metabolic deficiencies in which the body is unable to convert long-chain fatty acids into energy. The inability to produce energy from fat can lead to severe depletion of glucose in the body, causing patients to experience acute metabolic crises during times of increased energy demand, such as common infections or moderate exercise. These metabolic crises may manifest as serious liver, muscle and heart disease, and can lead to hospitalizations or early death. LC-FAOD are included in newborn screening panels across the U.S. and in certain European countries. Patients with LC-FAOD are currently managed with the avoidance of fasting, low-fat/high carbohydrate diets, carnitine, and medium-chain triglyceride (MCT) oil, a medical food product. Despite current management, many patients have significant metabolic events including hospitalizations and mortality due to LC-FAOD.
About UX007 (triheptanoin)
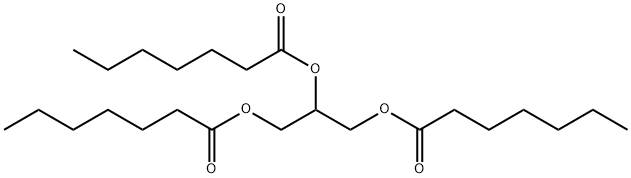
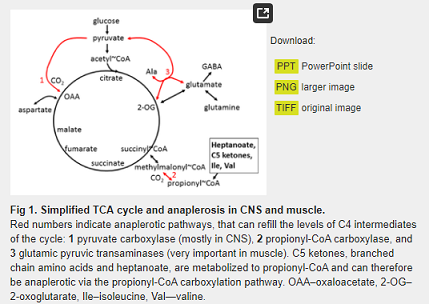
UX007 is a highly purified, pharmaceutical-grade, medium-chain triglyceride consisting of three 7-carbon fatty acids on a glycerol backbone created via a multi-step chemical process. It is an investigational therapy that directly addresses the deficiencies in LC-FAOD by providing patients with an alternative energy source that can be metabolized to increase intermediate substrates in the Krebs cycle, a key energy-generating process. Unlike typical even-chain fatty acids, one of the Krebs cycle intermediates generated specifically by UX007, also can be converted to new glucose, potentially providing an important added therapeutic effect, particularly when glucose levels are too low.
About Ultragenyx Pharmaceutical, Inc.Ultragenyx is a biopharmaceutical company committed to bringing patients novel products for the treatment of serious rare and ultra-rare genetic diseases. The company has built a diverse portfolio of approved therapies and product candidates aimed at addressing diseases with high unmet medical need and clear biology for treatment, for which there are typically no approved therapies treating the underlying disease.
The company is led by a management team experienced in the development and commercialization of rare disease therapeutics. Ultragenyx's strategy is predicated upon time and cost-efficient drug development, with the goal of delivering safe and effective therapies to patients with the utmost urgency
);You may like
Related articles And Qustion
See also
Lastest Price from TRIHEPTANOIN manufacturers

US $0.00-0.00/Kg2024-04-08
- CAS:
- 620-67-7
- Min. Order:
- 1Kg
- Purity:
- 99.9%
- Supply Ability:
- 200tons

US $20.00-1.00/kg2023-12-11
- CAS:
- 620-67-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons