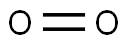Oxygen (O) - Chemical properties, Health and the lack of sufficient
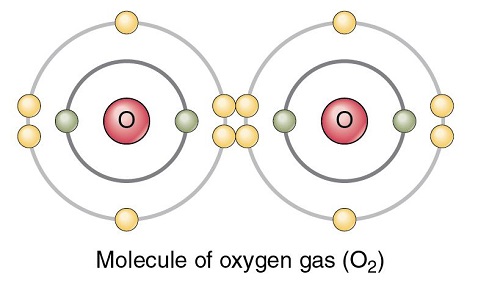
Oxygen is a very prevalent and important element and is necessary for sustaining life on this planet. This element is the third most abundant in mass behind helium and hydrogen in the universe. The diatomic form (O2) is the most common pure form. With a boiling point at -183 °C, O2 exists as a colorless and odorless gas at standard temperature and pressure. In the process of cellular respiration, the highly reactive O2 is used as the oxidant in breaking down food molecules to produce energy. In turn, photosynthetic organisms generate O2 by using energy from the sun and water. Other allotropes of pure oxygen exist, including the trioxygen (O3) form known as ozone, as well as other, less common allotropes of oxygen such as O4 and O8. These oxygen allotropes are formed under high pressure and low temperatures and are solid.

Uses
The most obvious use of oxygen is to support life. The process of aerobic respiration uses O2 in an oxidation reaction that produces H2O and energy required for metabolic reactions. Uses of commercially produced O2 gas are mainly industrial and medical. O2 is a highly reactive element that reacts readily with most other chemicals. The process of combustion uses oxygen as the oxidizing element; for instance, the combustion of hydrocarbons in cars requires O2 in a chemical reaction to produce H2O, CO2, and energy as byproducts. Similarly, rocket fuel containing H2 gas uses liquid O2 in a combustion reaction to produce water vapor and heat. O2 is used in a wide variety of commercial industries. Most commercially produced O2 is used in the production of steel from iron ore. O2 is used in the production of other metals as well, such as zinc, copper, and lead. O2 is used in the gasification of coal to promote more complete combustion in incinerators; in the production of various chemicals such as ethylene oxide, propylene oxide, and nitric acid; as a fuel component in blow torches for the welding and cutting of metals. i.e., oxyacetylene torch; and in the papermaking industry to bleach pulp. O2 is used in wastewater treatment facilities and to reduce hydrogen sulfide in sewers. O2 has many therapeutic uses in medicine. Highpurity O2 gas is used in life support in surgeries, in intensive care units to assist breathing, for premature babies, and with inhalation therapies with chronic conditions. Hyperbaric oxygen chambers provide oxygen at high partial pressures for numerous conditions, including instances of CO poisoning or in treatment for decompression sickness, or ‘the bends,’ in divers.
Mechanism of Toxicity
The lack of sufficient O2 delivery to tissues of the body and thus insufficient supply of energy to support cellular processes is known as hypoxia. The partial pressure of O2 that exists in air is 160mmHg, and hypoxic effects will start to occur at partial pressures of 120 mmHg and below. Occupational Safety and Health Administration standards require 19.5% oxygen. The brain, with a high metabolic rate and no O2 reserve, is the most susceptible to hypoxia. Complex physiological changes occur in response to hypoxia, including an increase in heart and respiratory rate and artery constriction, all to try to ensure adequate tissue oxygenation. Depending on the rate and extent of hypoxia onset, different symptoms will be prevalent with loss of consciousness and cessation of respiration and heart activity in extreme states of hypoxia below about 10% O2. Hypoxia due to carbon monoxide poisoning is due to the higher relative affinity of hemoglobin in red blood cells to CO over O2, resulting in insufficient O2 delivery to metabolizing tissues, with deleterious effects on the heart and the central nervous system (CNS) being most problematic. The partial reduction of molecular oxygen in biological systems produces cytotoxic intermediates, including the superoxide, hydrogen peroxide, and hydroxyl radical. The superoxide radical especially plays a significant role in a number of pathophysiologic states associated with oxygen toxicity. Reactive oxygen species (ROS) cause lipid peroxidation in the cell membranes, leading to cellular damage and death. The ROS generated can also lead to the production of other free radicals like nitric oxide, peroxynitrite, and trioxidane, which can cause damage to DNA and other molecules. Oxygen radical scavengers such as superoxide dismutase and catalase protect the body against oxygen-free radicals produced in day-to-day metabolic reactions.
Environmental Fate
Atmospheric air contains 20.8% O2. Despite consumption of O2 through respiration and oxidative processes, this concentration remains constant, most likely due to the depleted O2 being replaced by plant-generated O2 in the photosynthetic process. O2 does not bioaccumulate in organisms as pure oxygen.
);