Perfluorotributylamine: Overview, Potential in Tumor Treatment and Toxicity
General Description
Perfluorotributylamine, commonly used in the semiconductor industry for its stability, faces environmental and health concerns due to persistence and bioaccumulation. Its inertness and thermal resistance make it valuable in electronics manufacturing and tumor treatment, where it shows promise in downregulating platelet-derived TGFβ to inhibit metastasis. However, studies on animals have revealed toxic effects, including ocular damage and liver enzyme disruption. Ongoing research is crucial to understand its implications fully. Despite its industrial value, regulatory efforts are necessary to address potential drawbacks and explore alternative compounds with lower environmental impact.

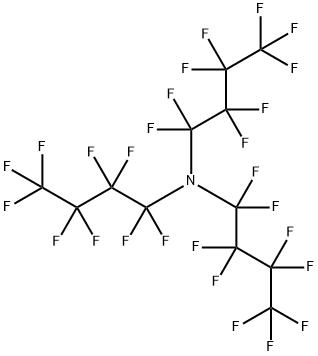
Figure 1. Perfluorotributylamine
Overview
Perfluorotributylamine is a synthetic compound extensively utilized in the semiconductor industry due to its exceptional stability and resistance to chemical degradation. Its fully fluorinated carbon backbone provides thermal stability and low volatility, making it an ideal processing aid for high-precision manufacturing processes. However, the environmental and health risks associated with Perfluorotributylamine's persistence and bioaccumulation have led to strict regulations in many countries. As a perfluorinated compound, Perfluorotributylamine consists of carbon, fluorine, and nitrogen atoms arranged with three butyl groups and an amine functional group. Its inertness and thermal resistance have enabled its use in electronics manufacturing, pharmaceuticals, and as a calibration standard in mass spectrometry. Despite its industrial value, concerns about PFTBA's environmental impact as a potential greenhouse gas with a long atmospheric lifetime are driving research efforts to mitigate its effects and explore alternative compounds with similar performance benefits but lower environmental risks. In conclusion, while Perfluorotributylamine offers significant advantages in industrial applications, its environmental implications underscore the importance of ongoing research and regulation to address its potential drawbacks. 1
Potential in tumor treatment
Perfluorotributylamine shows promising potential in tumor treatment by downregulating platelet-derived transforming growth factor-β (TGFβ) to inhibit tumor metastasis. Tumor metastasis is a significant factor in the low survival rates of cancer patients, with TGFβ playing a crucial role in promoting metastasis. Platelets contain high levels of TGFβ, which are released into the bloodstream upon activation, making them a key source of circulating TGFβ. Through the use of unfolded human serum albumin-coated Perfluorotributylamine nanoparticles, researchers have demonstrated effective downregulation of platelet-derived TGFβ both in vitro and in blood plasma. This downregulation resulted in the inhibition of epithelial-mesenchymal transition in tumor cells, reduced migration and invasion capabilities, and improved immune surveillance by NK cells. In animal models of various metastatic cancers, including colon cancer, melanoma, and breast cancer, intravenous administration of Perfluorotributylamine nanoparticles led to a significant reduction in tumor metastasis and improved survival rates. Compared to conventional antiplatelet drugs like ticagrelor, Perfluorotributylamine nanoparticles showed a superior therapeutic benefit with reduced bleeding risks, making them a promising candidate for preventing tumor metastasis. In conclusion, the downregulation of platelet-derived TGFβ by Perfluorotributylamine nanoparticles represents a potential and effective approach in cancer therapy. 2
Toxicity
Perfluorotributylamine has been investigated as a potential vitreous substitute due to its heavier density compared to saline. However, studies on laboratory animals have revealed several toxic effects associated with Perfluorotributylamine exposure. When injected into the vitreous space of rabbits, Perfluorotributylamine dispersed into smaller droplets, leading to the formation of cell clusters in the upper vitreous and on the lens surface. Histopathological changes included irregular defects in outer segment disks and a cellular response dominated by monocyte-derived macrophages capable of ingesting fluorochemicals. Moreover, replacing the vitreous body with Perfluorotributylamine in rabbit eyes resulted in degeneration of the outer segment layer, damage to the inner segment and RPE layers, and progressive deterioration in eyes with prolonged exposure. Studies in mice demonstrated that Perfluorotributylamine had a slight inhibitory effect on the liver's cytochrome P-450 system initially, followed by stimulation and return to baseline levels after 30 days. Additionally, intravenous infusion of dispersed Perfluorotributylamine in rabbits caused thrombocytopenia, clotting abnormalities, and potential coagulation inhibitor presence. Further research is needed to fully comprehend the implications of these toxic effects in human applications. 3
Reference
1. ANGELA C. HONG. Perfluorotributylamine: A novel long-lived greenhouse gas. Geophysical Research Letters. 2013, 40(22): 6010-6015.
2. Luo L, Zhang B, Tao F, et al. Perfluorotributylamine-Loaded Albumin Nanoparticles Downregulate Platelet-Derived TGFβ to Inhibit Tumor Metastasis. ACS Nano. 2023, 17(16): 15388-15400.
3. Perfluorotributylamine. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 9397.
);You may like
Related articles And Qustion
Lastest Price from Perfluorotributylamine manufacturers
US $8.00-0.80/KG2024-04-09
- CAS:
- 311-89-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $20.00-9.00/kg2023-12-11
- CAS:
- 311-89-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons