Pharmacodynamics and toxicology of Phenibut
Phenibut (β-phenyl-γ-aminobutyric acid) is an analog of the inhibitory neurotransmitter GABA (γ-aminobutyric acid), with potent psychotropic effects. It was first synthesized in St. Petersburg, Russia, in the early 1960s, and was at one time placed in the medical kits provided to Soviet cosmonauts [1]. The drug has been sold under the brand names of Noofen and Anvifen, and produces a wide range of psychological effects, including sedation and purported nootropic (cognitive enhancing) effects. Phenibut is not available for prescribed use in the USA, European Union, or Australia, although it can be legally purchased as a dietary supplement through a variety of online vendors [2]. The drug acts predominately as a GABAB agonist, although other receptor systems may be involved [1].

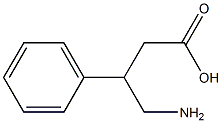
Figure 1 The structure of Phenibut
Outside of Russia and the former Soviet Republics, phenibut was relatively unknown until 2011, when a quantity of the drug was seized in Sweden, and the European authorities were alerted [2, 3]. Due to concerns for potential misuse, phenibut was reported to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) and Europol in 2012 [2]. In the EMCDDA–Europol 2012 Annual Report on the implementation of Council Decision, it was noted that phenibut was “being sold both as a ‘dietary supplement’ and ‘research chemical’ in a number of EU Member State” [4]. Following the notification to EMCDDA, phenibut was classified as a new psychoactive substance (NPS) in 2012 by the United Nations Office of Drug and Crime (UNODC). NPS is defined by UNODC as: “a substance of abuse, either in a pure form or a preparation, that are not controlled by the 1961 Single Convention on Narcotic Drugs or the 1971 Convention on Psychotropic Substances, but which may pose a public health threat” [2, 3].
Pharmacology and mechanism
General effects on the central nervous system: systemically administered PB produces a great variety of central effects. At doses that do not affect motor activity (e.g., 20 mgGkg i.p.) PB inhibits food conditioned reflexes in mice. At doses higher than 70 mgGkg i.p. PB reduces motor and exploratory activities, rearings, muscle tone, coordination and body temperature. It potentiates central effects of the anesthetics: ether, chloral hydrate, and barbiturates. Its primary mechanism of action has been reported as a GABAB receptor agonist, although its interaction with multiple other receptor systems has been de- scribed [1].
Structurally, phenibut is a GABA molecule with a phenyl group attached at the beta carbon. The addition of this phenyl group gives the drug a greater permeability through the blood brain barrier compared to GABA; however, it does not provide more potent pharmacological effects [1]. The addition of a single chlorine atom to the phenyl ring of phenibut produces the spasmolytic drug baclofen (β-p-Cl-phenyl-γ- aminobutyric acid). Thus, strong structural commonalities exist between GABA, baclofen, and phenibut [1].
GABA is the primary inhibitory neurotransmitter in the CNS and produces effects through the activation of two mechanically different receptors: GABAA and GABAB [5, 6]. The GABAA receptor is an ionotropic ligand-gated chloride channel and provides rapid neuronal inhibition [6]. The receptor is the target for many sedative/hypnotic drugs, including benzodiazepine, barbiturates, and the non-benzodiazepine
Z drugs, such as zolpidem. The GABAB receptor, contrarily, is a metabotropic G protein–coupled receptor, which mediates slow and protracted inhibitory neurotransmission in the CNS, and maintains an inhibitory tone [5]. Baclofen, which is used to treat muscle spasticity in several neurological disorders such as multiple sclerosis, is a GABAB receptor agonist [5, 6].
Pharmacodynamics and toxicology
Very little information is available on the pharmacokinetics or toxicology of PB in animals. Following intravenous administration to either rabbits or rats PB is not metabolized. PB is largely excreted in the urine. At 15, 30, 60, or 90 min following i.v. administration PB was found in liver, kidneys, and urine [7]. Traces of PB (~4 mg%) were found in blood and brain. 180 min after i.v. injection only traces of the drug were found in all tissues studied. In vitro tissue binding studies indicated that PB binds to liver, kidney, and brain tissue. In cats and dogs PB, after a single dose of 50 mgGkg i.v., is excreted in the urine unchanged.
The acute toxicity of PB is low. Its LD50 is 900 mgGkg i.p. in mice and 700 mgGkg i.p. in rats [7].
Treatment and clinical use
PB is widely used in Russia in different neurological and psychiatric disorders. In some Russian reference books PB is listed as a tranquilizer, in others as a nootropic agent. In reality, the drug appears to have both types of activity. It has been reported to diminish tension, alleviate anxiety and fear, and to potentiate neuroleptics and antiparkinsonian drugs (7). It has also been reported to enhance memory and intellectual function. The largest amount of information (7) is available on the clinical use of PB in neuroses (i.e., mental disorders characterized by anxiety). In geriatric patients, PB appears to be superior to tranquilizers or neuroleptics. Like BAC, PB reduces spasticity. It has been successfully used in the treatment of post-traumatic stress disorder, “asthenic-depressive” syndrome, stuttering, and even vestibular disorders.
In children, PB has been claimed to be effective in neurotic disorders, “organic brain syndrome,” insomnia and various forms of hyperactivity. In preschool age children PB has been successfully used for speech disorders, particularly stuttering (7).
The first step in treatment in phenibut use is to identify the underlying clinical concern or diagnosis. It is important for clinicians to keep in mind that a presentation involving excessive phenibut use does not necessarily infer a diagnosis of a substance use disorder (addiction). Although that may be the case, it is imperative to thoroughly assess the behavioral patterns and screen for any underlying mental health difficulty. It is not uncommon for patients to misuse substances as a means of self-soothe emotional distress, in the absence of addiction. Thus, clinicians should evaluate for any mood or anxiety dis- orders and implement the appropriate therapies if such an ailment becomes recognized.
If phenibut addiction is present or highly suspected, the patient should be referred to a substance use treatment facility for further evaluation. There are no specific treatments designated specifically for phenibut addiction, and patients should be treated with the traditional evidence-based SUD psycho- therapies along with supportive pharmacotherapies, if indicated.
Given the propensity of phenibut to precipitate states of physical dependence and withdrawal, a medically supervised detoxification may be necessary. Substituting phenibut with either baclofen or phenobarbital has been successfully used in detoxification, as previously stated [8, 9]. Having the patient self-taper, their use of phenibut is not recommended for many reasons, but namely because it hinders the clinician’s ability to accurately track the amount of drug being used during the tapering process. Additionally, given that most clinicians have no experience prescribing or monitoring phenibut, they will be unfamiliar with dosage requirements, pharmacokinetic or pharmacodynamic properties, and general med management strategies. Once the patient has completed an initial addiction treatment program, they should be monitored regularly over the long term, in view of addiction as a chronic relapsing- remitting disease.
References
1. Lapin I. Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug. CNS Drug Rev. 2001;7(4):471–81.
2. Van Hout MC. A narrative review of the naturally occurring inhibitory neurotransmitter gamma-aminobutyric acid (GABA) called phenibut in dietary supplements. Perform Enhance Health. 2018;6(1):33–5.
3. Owen DR, Wood DM, Archer JR, Dargan PI. Phenibut (4-amino-3- phenyl-butyric acid): availability, prevalence of use, desired effects and acute toxicity. Drug Alcohol Rev. 2016;35(5):591–6.
4. EMCDDA-Europol. New drugs in Europe, 2012, EMCDDA- Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA. Luxembourg: Publications Office of the European Union; 2013
5. Frangaj A, Fan QR. Structural biology of GABAB receptor. Neuropharmacology. 2018;136(Pt A):68–79. https://doi.org/10. 1016/j.neuropharm.2017.10.011.
6. Jacobson LH, Vlachou S, Slattery DA, Li X, Cryan JF. The gamma- aminobutyric acid B receptor in depression and reward. Biol Psychiatry. 2018;83(11):963–76.
7. Lapin I, Phenibut (beta-phenyl-GABA): a tranquilizer and nootropic drug. [J]. Cns Drug Reviews, 2010, 7(4):471-481.
8. Brunner E, Levy R. Case report of physiologic phenibut dependence treated with a phenobarbital taper in a patient being treated with buprenorphine. J Addict Med. 2017;11(3):239–40.
9. Samokhvalov AV, Paton-Gay CL, Balchand K, Rehm J. Phenibut dependence. BMJ Case Rep. 2013:bcr 2012008381.
);You may like
See also
Lastest Price from Phenibut manufacturers

US $10.00/ASSAYS2024-04-27
- CAS:
- 1078-21-3
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 100kg

US $4.00-250.00/g2024-04-27
- CAS:
- 1078-21-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg