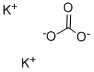
Potassium carbonate: Preparation, application, quantitative method and toxicity

Potassium carbonate is prepared commercially by the reaction potassium hydroxide with carbon dioxide [1]. From the solution crystallizes the sesquihydrate K2CO3·3H2O ("potash hydrate"). Heating this solid above 200 °C gives the anhydrous salt. In an alternative method, potassium chloride is treated with carbon dioxide in the presence of an organic amine to give potassium bicarbonate.
Quantitative method
The content of potassium carbonate can be determined by titration. In a bottle with a stopper, accurately weigh 1g of the dried sample obtained by the "loss on drying" method and dissolve it in 50mL of water. After adding 2 drops of methyl red test solution (TS-149), slowly titrate with 1 mol/L hydrochloric acid under constant stirring until the solution turns pale pink. Heat the solution until it boils and cool. Continue titrating until the light pink color no longer disappears after boiling. Each 1mol/L hydrochloric acid is equivalent to 69.10 mg of potassium carbonate.
Toxicity
Exposure of rabbits to potassium carbonate emulsion at 50 mg/L and 100 mg/L via oral drinking for 14 consecutive days caused significant increase in the creatinine and uric acid at 100 mg/L by 48.6% and 126.3% respectively. Also, potassium carbonate emulsion significantly increased serum blood urea nitrogen at 50 mg/L and 100 mg/L concentration. The results suggested that potassium carbonate emulsion exposure via oral drinking could precipitate kidney damage [3].
[2]Leonard, J.; Lygo, B.; Procter, G. Advanced Practical Organic Chemistry 1998, Stanley Thomas Publishers Ltd.
[3]Akintunde et al. Exposure to potassium carbonate emulsion induced nephrotoxicity in experimental animals. Jordan journal of biological science. 2010, 3(1): 29-32.
);You may like
Related articles And Qustion
Lastest Price from Potassium carbonate manufacturers

US $1340.00-1330.00/tons2024-04-28
- CAS:
- 584-08-7
- Min. Order:
- 10tons
- Purity:
- 99%
- Supply Ability:
- 100tons

US $10.00/kg2024-04-27
- CAS:
- 584-08-7
- Min. Order:
- 1kg
- Purity:
- 99.7%
- Supply Ability:
- 200000kg