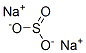Preparation and Applications of Sodium sulfite
Sodium sulfite is a white, water-soluble, crystalline solid with a sulfurous, salty taste. It decomposes when heated. It is generally available f in powder, crystalline, and tablet forms.
Sodium sulfite is a dechlorinating agent widely used by utilities. It is generally available in powder form. In addition, some companies manufacture in tablet form. Sodium sulfite is slightly alkaline in nature. Sodium sulfite is a reducing agent and is reported to scavenge more oxygen than sodium thiosulfate.

Sodium Sulfite Preparation
1. Sodium sulfite is generally prepared in laboratories from the reaction between sodium hydroxide (NaOH), and gaseous sulfur dioxide (SO2). The chemical equation for this reaction can be given as follows:
SO2 + 2NaOH → Na2SO3 + H2O
The NaOH reactant depletion can be detected through the addition of a few drops of concentrated H2SO4, resulting in the SO2 gas liberation.
2. Na2SO3 is produced on the industrial basis from the reaction between sodium carbonate and sulfur dioxide solution. Initially, sodium bisulfite (NaHSO3) compound is formed. Now, this resultant compound reacts either with sodium hydroxide or sodium carbonate to yield sodium sulfite product. The reaction can be generalized as follow:
Na2CO3 + SO2 → Na2SO3 + CO2
Applications
Sodium sulfite is primarily used in the pulp and paper industry.It has been also applied in the thermomechanical conversion of wood to fibres (defibration) for producing medium density fibreboards (MDF).
As an oxygen scavenger agent, it is used to treat water being fed to steam boilers to avoid corrosion problems,in the photographic industry, it protects developer solutions from oxidation and (as hypo clear solution) to wash fixer (sodium thiosulfate) from film and photo-paper emulsions.
As a reducing agent it is used in the textile industry as a bleaching, desulfurizing, and dechlorinating agent (e.g. in swimming pools). Its reducing properties are exploited in its use as a preservative to prevent dried fruit from discoloring, and for preserving meats.
It is used as a reagent in sulfonation and sulfomethylation agent. It is used in the production of sodium thiosulfate.
);You may like
See also
Lastest Price from Sodium sulfite manufacturers

US $1.80-1.40/kilograms2024-04-30
- CAS:
- 7757-83-7
- Min. Order:
- 1kilograms
- Purity:
- 99%
- Supply Ability:
- 1000tons
US $397.00-300.00/yon2024-04-30
- CAS:
- 7757-83-7
- Min. Order:
- 1yon
- Purity:
- 99%
- Supply Ability:
- 80ton