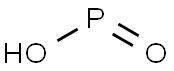
Preparation of Hypophosphorous acid
Background
Hypophosphorous acid is the starting product for the synthesis of organophosphorus compounds. Hypophosphorous acid is used in the synthesis of compounds containing the P-C bond [I], and also organo[1]phosphorus bifunetional compounds [2]. The compounds obtained from hypophosphorous acid have bio[1]logical activity [3], are surface-active agents, antioxidants and flotation agents [4], are used in photography to obtain developers that do not darken with time [5], etc.

Picture 1 Hypophosphorous acid aqueous solution
Preparation
Up to now the isolation of hypophosphorous acid in a chemically pure state and in adequate amount is associated with great difficulties. The known methods consist in treating aqueous solutions of hypophosphites, purified by various procedures, with acids (sulfuric, oxalic) [6]. The precipitate is filtered, and the aqueous solution is evaporated using various precautions. In some cases, after complete evaporation of the solution, the residual salts dissolved in the obtained acid are removed by precipitation with alcohol[1]. However, none of these methods make it possible to completely remove trace amounts of impurities. Evaporation of tile aqueous solution of hypophosphorous acid is accompanied by oxidation to phosphorous and phosphoric acid~ and also by dis[1]proportionation to hydrogen phosphide [phosphine] and phosphorous acid, which cannot be removed from the desired product. Besides this, the slightest excess of one of the starting products makes it impossible to remove it from the reaction mixture. The acid is obtained as a viscous liquid that fails to crystallize. Special methods, making it possible to obtain crystalline hypophosphorous acid in the pure state, are laborious and give a low yield.
Properties
Hypophosphorous acid is slightly soluble in diethyl ether. This caused us to try extracting the hypo[1]phosphorous acid, obtained by known methods [6, 8], with diethyl ether. To obtain the hypophosphorous acid we used commercial calcium hypophosphite and sulfuric acid without prior purification. The hypophosphorous acid, obtained as a viscous, faintly yellow syrup, was extracted in a liquid-liquid continuous extraction apparatus. The acid obtained in this manner was a colorless liquid, which crystallized when cooled slightly, and remained crystalline at room temperature. The yield of the acid was 80%; m.p. 23-25~ nD2~ 1.4601; d42~ 1.479. Found %: P 46.19, 46.24; equiv, wt. 66.55; MR 12.23. H4P205. Calculated %: P 46.93; equiv, wt. 66; ~ 11.83. The atomic refraction of phosphorus was taken as 4.79. Since phosphoric acid could be an impurity, the product was tested qualitatively for phosphate ion using ammonium molybdate[1]. A yellow precipitate or color was not observed. A method was found for the isolation of chemically pure hypophosphorous acid in the cI:ystalline state by its continuous extraction with diethyl ether.
Hypophosphorous acid as a hydride ion donor
Hypophosphorous acid is a protonating agent and relatively good hydride ion donor in ionic hydrogenation and hydrogenolysis. Ionic hydrogenation of hydrogenolysis requires proton and hydride ion donors. Hypophosphorus acid, H3PO 2 (I), is presumably both a proton donor and hydride ion donor. In order to check this hypothesis, we selected the ionic hydrogenation of l-methylcyclohexene (II). This compound readily undergoes ionic hydrogenation to give methylcyclohexane by the action of hydride ion donors such as Et3SiH and strong acids such as CF3CO2H [i]. However, 1-methyl[1]cyclohexene in the presence of (I) is not converted to methylcyclohexane at 40~ over 4 h with a ten-fold excess of acid. The lack of a reaction between (I) and (II) may be explained either by assuming that H3PO 2 is a poor proton donor or a poor hydride ion donor. The reaction of (I) with (II) does not proceed in the presence Of Et3SiH which is an active hydride ion donor. Thus, H3P02 is a poor proton donor. However, when CF3CO2H is added to a reaction mixture of (I) and (II) in order to increase the acidity of the medium, ~5% methylcyclohexane was found in the reaction products. Hence, the P-H bond may serve as a hydride ion donor[8].
Thermal Disproportionation of Hypophosphorous Acid
Thermal disproportionation of hypophosphorous acid was studied to find the reaction order, the rate constant and activation energy of the process, and also the temperature ranges in which the reaction rate is the highest[9].
Reference
I. R.H. Williams and L. A. Hamilton, J. Am. Chem. Soc., 77, 3411 (1955); U. S. Patent 2,957,931 (1960}; Chem. Abstrs., 5_55, I0317h (1961); Brit. Patent 660,918 (1951); Chem. Abstrs., 46, 8145b (1952).
2. German Patent 870,701 (1953); Chem. Abstrs., 52, 16291h (1958).
3. German Patent 1,005,063 (1957); Chem. Zentr., _1959, 16095; U. S. Patent 2,648,706 (1953); Chem. Abstrs., 48, 8252b (1954).
4. U.S. Patent 2,957,931 (1960); Chem. Abstrs., 55, I0317h (1961).
5. Belgian Patent 557,556 (1957); Chem. Abstrs., 51, 17545c (1957).
6. Handbook of Preparative Inorganic Chemistry [Russian translation from the German], p. 276, Editor-in-Chief G. Brauer, IL, Moscow (1956).
7. W. Jenkins and R. T. Jones, J. Amer. Chem. Soe., 74, 1353 (1952). 8. C. Marie, Compt. rend., 138, 1216 (1904).
8 Karpeiskaya, O.A., Belyi, A.A., Parnes, Z.N. et al. Hypophosphorous acid as a hydride ion donor. Russ Chem Bull 37, 392–394 (1988).
9 Shechkov, G.T., Pevneva, I.A. & Meshkova, O.A. Thermal Disproportionation of Hypophosphorous Acid. Russian Journal of Applied Chemistry 76, 1354–1355 (2003).
);You may like
See also
Lastest Price from Hypophosphorous acid manufacturers

US $10.00-5.00/kg2024-04-30
- CAS:
- 6303-21-5
- Min. Order:
- 1kg
- Purity:
- 99.5
- Supply Ability:
- 10 ton per month

US $10.00/kg2024-04-26
- CAS:
- 6303-21-5
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 1000000000 ton