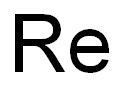Preparation of Rhenium
Rhenium [7440-15-5], chemical symbol Re, atomic number 75, and relative atomic mass 186.207(1), is the last metal of group VIIB(17) of Mendeleev’s periodic chart. The name comes from the Latin word Rhenus, meaning the German river Rhine. Rhenium is a silvery grayish white metal with a metallic luster; its density (21,020 kg.m–3) is exceeded only by that of platinum, iridium, and osmium, and its melting point of 3458 K (3185°C) is exceeded only by that of tungsten and carbon. Its crystal lattice structure is hexagonal close-packed (hcp) with a = 276.08 pm and c = 445.80 pm.
General Properties
From a mechanical point of view, rhenium is highly ductile and exhibits a high Young’s modulus (520 GPa) exceeded only by that of iridium and osmium and an elevated tensile strength (1170 MPa). Rhenium is a difficult metal to machine, and its formability is always a pitfall for the fabrication of parts having an intricate shape. Therefore, powder metallurgy techniques are suitable methods for fabricating rhenium parts.

Industrial Preparation
Rhenium is obtained commercially from molybdenum roaster-flue dusts obtained from the processing of copper-sulfide ores. During the roasting of molybdenite between 500 and 700°C, in order to yield the molybdenum-trioxide-evolving sulfur dioxide, rhenium sulfide oxidizes, forming above 250°C the volatile rhenium heptoxide (Re2O7) escaping with the dusty flue gases. The volatile compound is carried off in flue gases, absorbed in the scrubbing liquors, refined, and finally converted into ammonium perrhenate (NH4ReO4), also known by its commercial acronym, APR. Then, pure rhenium metal powder (99.99 wt.% Re) is obtained by thermal reduction of ammonium perrhenate with pure hydrogen at elevated temperatures.
The preparation is usually performed in a twostage process. In the first stage, ammonium perrhenate is thermally decomposed into ammonia and rhenium heptoxide. In the second stage, rhenium heptoxide is reduced to powdered metal by hydrogen gas. Afterwards, the rhenium powder is usually cold-isostatic pressed at 170 to 200 MPa and sintered in a hydrogen atmosphere at 75% of its melting point (i.e., 2480°C). By contrast, dense and high-purity rhenium metal (i.e., 99.995 wt.% Re) is obtained by remelting sintered compacts of rhenium by vacuum-arc remelting or electronbeam melting. The raw ingot of rhenium metal obtained can be further purified by zone refining.
You may like
Related articles And Qustion
See also
Lastest Price from RHENIUM manufacturers

US $6.00/KG2024-04-12
- CAS:
- 7440-15-5
- Min. Order:
- 1KG
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/Month

US $0.00-0.00/kg2023-12-25
- CAS:
- 7440-15-5
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 1000000