Procaine: a general anesthesia drug
Introduction
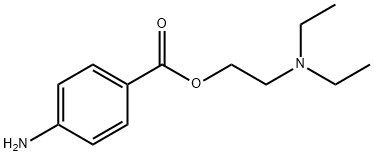
Procaine, a member of the amino ester class, stands as a widely employed local anesthetic, finding prominent use in medical and dental settings to induce temporary regional anesthesia during various procedures. Operating by inhibiting sodium ion influx into nerve cells, procaine effectively hampers nerve impulse transmission, providing localized pain relief. Typically administered via injections and sometimes complemented with substances like epinephrine to extend its effects, procaine's efficacy is notable for minor surgeries, though its duration of action remains limited. Acting by inhibiting the influx of sodium ions into nerve cells, procaine blocks nerve impulses, thereby alleviating pain in the targeted area. Administered primarily through injections, it is often combined with substances like epinephrine to enhance its effects. While effective for short-term pain relief in minor surgeries, procaine's duration of action is limited. Potential side effects include local reactions such as redness and swelling, with more severe allergic reactions being rare. Healthcare professionals consider patient medical history and contraindications when deciding on the suitability of procaine for an individual. Despite its historical use, newer and more potent local anesthetics are also available, and the choice of anesthetic depends on the specific requirements of the procedure and patient factors. While associated with common local reactions such as redness and swelling, severe allergic responses are infrequent. Healthcare professionals judiciously consider patient histories and contraindications in determining the appropriateness of procaine, recognizing its historical significance alongside the availability of newer, more potent local anesthetics tailored to specific procedural and patient needs1.
Application
An ideal general anesthetic should have the advantages of rapid onset, easy control of anesthesia depth, rapid and stable recovery, wide safety range, and fewer adverse reactions. At present, the main intravenous general anesthesia drugs used in clinical practice are propofol and etomidate. However, the use of propofol causes severe injection pain and has a significant inhibitory effect on the respiratory and circulatory systems; Although etomidate has minimal inhibitory effects on the respiratory and circulatory systems, it also has adverse reactions such as weak analgesic effects, injection pain, and adrenal cortex dysfunction. Procaine is a short-acting ester local anesthetic that has been used as an intravenous general anesthetic in combination with ketamine, diazepam, and succinylcholine for compound general anesthesia1. Procaine has good water solubility, stable chemical properties, and is non addictive. It has advantages such as fast wakefulness, good analgesic effect, and less discomfort after waking up when used in general anesthesia. However, it can cause dose-related methemoglobinemia and circulatory inhibition. It has disadvantages such as low anesthesia efficacy, small safety range, and difficult to control anesthesia depth.
Procaine is a commonly used antibiotic in equine medicine but its use is associated with a substantial incidence of adverse reactions. Soluble procaine concentrations were determined by HPLC in several commercially available procaine penicillin preparations, including some that were involved in adverse reactions. The mean (+/- SEM) soluble procaine concentrations in the veterinary preparations was 20.18 +/- 5.07 mg/ml, which was higher than the concentration in the only procaine penicillin preparation for use in humans in Australia of 7.3 mg/ml. Heating the veterinary procaine penicillin preparations to 50 degrees C for 1 day led to a significant (P less than 0.01) increase in the amount of soluble procaine. Heating to 50 degrees C for 7 days also produced a significant (P less than 0.02) increase. Soluble procaine tended to return to baseline concentrations when veterinary procaine penicillin preparations were heated to 50 degrees C for 2 days then stored for 7 days at room temperature. Administration of procaine HCl intravenously (IV) at 2, 5, and 10 mg/kg produced behavioural, locomotor and vascular reactions, which were clinically similar to those reported in adverse reactions to procaine penicillin. The more severe reactions occurred at higher doses, although different horses responded variably at the same dose. Some adverse reactions lead to recumbency but none were fatal. The blood procaine concentrations 1 min after IV administration averaged 19.0 +/- 12.6 and 25.3 +/- 16 micrograms/ml at 2.5 mg/kg and 5 mg/kg, respectively. Ten min after administration, blood procaine concentrations were significantly higher in the 5 mg/kg group than in the 2.5 mg/kg group. Intramuscular (IM) procaine HCl at 5 mg/kg produced significantly lower (P less than 0.001) blood concentrations than similar IV doses, and, in contrast to the IV doses, the amount of procaine in the blood was significantly higher 5 and 10 min after administration than it was after 1 min. Mild excitatory reactions in 4/5 horses were noted 5 to 10 min after IM administration. Administration of diazepam 20 s before procaine HCl prevented the excitatory adverse reaction in 2/2 horses, but administration after the procaine did not influence the outcome2.
Synthesis
The synthesis route design of procaine derivatives Procaine is a compound obtained by reacting - bromoethyl 4- ((tert butoxycarbonyl) amino) benzoate (intermediate II) with diethylamine Based on previous research and literature reports, a reasonable molecular design approach was employed to modify its chemical structure using the procaine framework. After reacting intermediate II with three different amines, three new compounds were obtained, named PL18, PL19, and PL20 in sequence. Confirm the structure of the target compound through nuclear magnetic resonance hydrogen spectroscopy, nuclear magnetic resonance carbon spectroscopy, and high-resolution mass spectrometry. The synthesis route is shown in Figure 1. Three procaine derivatives PL18, PL19, and PL20 were designed and prepared through side chain modification. The larger the distribution coefficient of lipid and water, the better the lipid solubility, and the stronger the ability of drugs to penetrate the blood-brain barrier, which may result in stronger drug efficacy. The lipid water distribution coefficients of the three compounds prepared gradually increased, and the half effective dose (ED50) gradually decreased, consistent with speculation3.
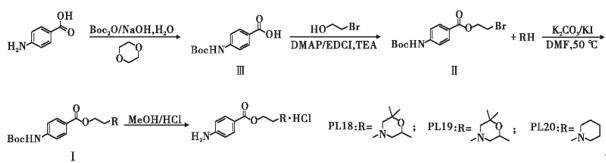
Figure 1. Synthesis of Procaine
Safety
Procaine is the most widely used antibiotic in equine medicine and is also used extensively in other animal species. Its use in human medicine has declined over recent years as penicillins with broader antibacterial activity and superior pharmacokinetic properties have become available. Procaine penicillin is usually extremely well tolerated in animals and man, yet there have been reports of adverse reactions and, occasionally, deaths after IM injections of therapeutic doses of the drug in horses and man. The causes of procaine penicillin toxicity are believed to include anaphylaxis to penicillin, penicillin toxicity, procaine toxicity, procaine penicillin psychosis. toxicity from breakdown products or excipients in the formulations, and the consequences of drug-based vascular emboli. While there has been a belief that anaphylaxis to penicillin is the predominant cause of adverse reactions in horses. Animal experiments were conducted to evaluate the general anesthesia effect and awakening quality of the compound. Based on both mouse and rat experiments, Procaine-19 (Figure 1;PL19) anesthesia has a relatively small ED50 (i.e. better efficacy) and a relatively high therapeutic index, with a wide safety range. During the continuous infusion process, compared with procaine, Procaine-19 has little effect on the respiratory rate of rats. The anesthesia depth during the infusion process is controllable, and the awakening is fast and of good quality after discontinuation. There are fewer adverse reactions such as convulsions and muscle rigidity, and there is no significant impact on the feeding water, liver function, kidney function, and heart of rats. Procaine-19 has good drug efficacy and can be used as a new type of general anesthesia drug.
Reference
1. Chapman CB, Courage P, Nielsen IL, Sitaram BR, Huntington PJ. The role of procaine in adverse reactions to procaine penicillin in horses. Aust Vet J. 1992 Jun;69(6):129-33.
2. Stevenson AJ, Weber MP, Todi F, Mendonca M, Fenwick JD, Young L, Kwong E, Chen F, Beaumier P, Timmings S, et al. Determination of procaine in equine plasma and urine by high-performance liquid chromatography. J Anal Toxicol. 1992 Mar-Apr;16(2):93-6.
3. Zhao Yi, Yin Jiaqi, Hai Ao et al. Design, synthesis, and biological evaluation of procaine derivatives [J]. Huaxi Pharmaceutical Journal, 2022, 37 (05): 485-489.
);You may like
Related articles And Qustion
See also
Lastest Price from Procaine manufacturers

US $1.00/g2024-04-27
- CAS:
- 59-46-1
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $1.00/g2024-04-25
- CAS:
- 59-46-1
- Min. Order:
- 1g
- Purity:
- 0.98
- Supply Ability:
- 1000