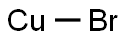Properties and Preparation of Copper(I) bromide
Copper(I) bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds and as a lasing medium in copper bromide lasers.

Uses
Copper(I) Bromide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered. Most metal bromide compounds are water soluble for uses in water treatment, chemical analysis and in ultra high purity for certain crystal growth applications. The bromide ion in an aqueous solution can be detected by adding carbon disulfide (CS2) and chlorine. American Elements produces to many standard grades when applicable, including Mil Spec (military grade); ACS, Reagent and Technical Grade; Food, Agricultural and Pharmaceutical Grade; Optical Grade, USP and EP/BP (European Pharmacopoeia/British Pharmacopoeia) and follows applicable ASTM testing standards. Typical and custom packaging is available.
Properties and Preparation
The compound is white, although samples are often colored due to the presence of copper(II) impurities.The copper(I) ion also oxidizes easily in air. It is commonly prepared by the reduction of cupric salts with sulfite in the presence of bromide.For example, the reduction of copper(II) bromide with sulfite yields copper(I) bromide and hydrogen bromide:
2 CuBr2 + H2O + SO32- → 2 CuBr + SO42- + 2 HBr
CuBr is insoluble in most solvents due to its polymeric structure, which features four-coordinated, tetrahedral Cu centers interconnected by bromide ligands (ZnS structure). Upon treatment with Lewis bases, CuBr converts to molecular adducts. For example, with dimethyl sulfide, the colorless complex is formed:
CuBr + S(CH3)2 → CuBr(S(CH3)2)
In this coordination complex, the copper is two-coordinate, with a linear geometry. Other soft ligands afford related complexes. For example, triphenylphosphine gives CuBr(P(C6H5)3), although this species has a more complex structure. Thermal excitation of copper(I) bromide vapour yields a blue-violet emission which is of greater saturation than known copper(I) chloride emission.Copper(I) bromide is hence an advantageous emitter in pyrotechnic flames.
);You may like
Related articles And Qustion
Lastest Price from Cuprous bromide manufacturers

US $0.00/kg2023-12-11
- CAS:
- 7787-70-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons

US $30.00/L2023-10-08
- CAS:
- 7787-70-4
- Min. Order:
- 1L
- Purity:
- 99%
- Supply Ability:
- 20T