Reaction / Application on Synthetic Works of this Intermediate
(S)-22-(tert-butoxycarbonyl)-43,43-dimethyl-10,19,24,41-tetraoxo-3,6,12,15,42-pentaoxa-9,18,23-triazatetratetracontanoic acid is an important organic intermediate to act as crosslinkers to functionalize molecules.
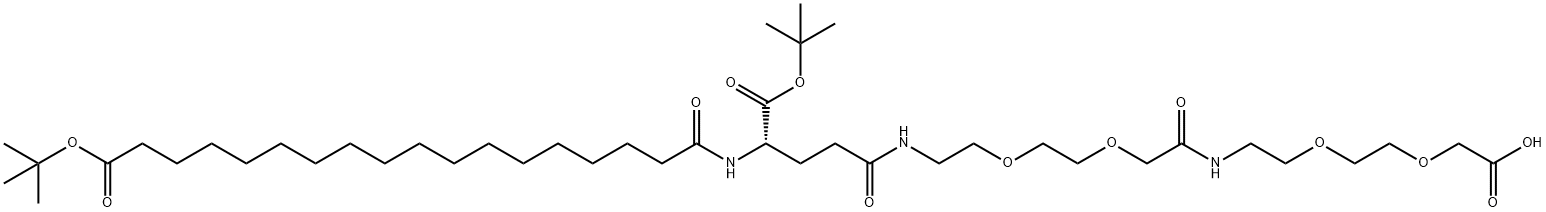
The following example is about its application on the synthesis of Dchbs-active esters of PEG compounds[1]
t-Bu protected C18-diacid-yGlu-Ado-Ado-OH (0.461mmol, 0.390g), 3,5-dichloro-2-hydroxy-N,N-dimethyl-benzenesulfonamide (0.507mmol, 0.137g, l. leq) and DCC(0.553mmol, 0.114g, 1.2eq) was dissolved in 1.5mL DCM. Solution was stirred at RT for 18 hours. The reaction mixtures were analyzed by UPLC. Extra DCC (0.340 mmol, 0.07g, 0.7eq) was added and the reaction was stirred at RT for another 18 hours. The conversion was ~57 percent when the DCU was removed by filtration. The supernatant was washed with brine and dried over Mg2SO4. The crude was purified using silica gel column chromatography with a gradient eluent from DCM to 5percent MeOH in DCM. After evaporation the product yield was 307 mg (61percent). TFA was used to cleave the tBu-esters for 1.5 hours. The cleavage mixture was evaporated under reduced pressure. The compound was twice dissolved in MeCN and evaporated. The sticky oil was triturated in diethyl ether. A white precipitate was obtained. Yield of product (0.198g, 72 percent).
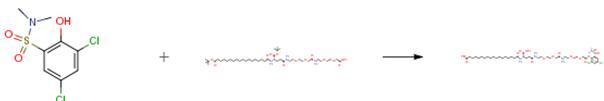
The following example is about its application on the synthesis of acylated insulin analogues [2]
The tert-butyl octadecandioyl-Glu(OEG-OEG-OH)-OtBU (0.63 g) was dissolved in THF (35 ml). DIEA (0.255 ml, 2 eq.) was added followed by TSTU (0.45 g, 2 eq.), and the mixture was stirred at room temperature for 16 hours. The mixture was partitioned between ethyl acetate (250 ml) and aqueous NaHSO4 (3×100 ml). The organic phase was dried (MgSO4) and concentrated in vacuo to afford 0.65 g of 174 (S)-1-tert-butoxycarbonyl-3-{2-[2-({2-[2-(2,5-dioxopyrrolidin-1-yloxycarbonylmethoxy)ethoxy]ethylcarbamoyl}methoxy)ethoxy]ethylcarbamoyl}propylcarbamoyl)heptadecanoic acid tert-butyl ester (alternative name: tert-butyl octadecandioyl-Glu(OEG-OEG-OSu)-OtBu) as an oil.
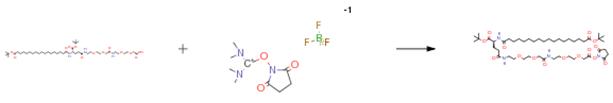
The following example is about its application on the synthesis of modulators of neuropeptide receptors [3].

To a solution of (S)-22-(tert-butoxycarbonyl)-43,43-dimethyl-10,19,24,41-tetraoxo -3,6,12, 15,42-pentaoxa-9,18,23-triazatetratetracontan-1-oic acid (Intermediate 2 (16)) (54.0 mg, 0.063 mmol), N-hydroxysuccinimide (14.6 mg, 0.127 mmol), and HATU (24.1 mg, 0.063 mmol) in 1.0 ml of DMF was added DIEA (0.022 ml, 0.127 mmol) and the product stirred for 30 mins at RT and used directly in the next step without further purification.
The following example is about its application on the synthesis of analogues with fatty acid substituents [4].
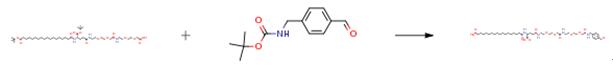
(4-Formyl-benzyl)-carbamic acid tert-butyl ester (Boc-aminomethylbenzaldehyde, 1 .54 g, 6.60 mmol) was dissolved in dichloromethane (50 mL) and solution of hydrochloric acid in dioxane (3.8 M, 20 mL, 76 mmol) was added. The mixture was stirred for 16 h and solid material precipitated from the solution. All solvents were removed by evaporation. 17-{(S)-1 -tert-Butoxycarbonyl-3-[2-(2-{[2-(2- carboxymethoxy-ethoxy)-ethylcarbamoyl]-methoxy}-ethoxy)-ethylcarbamoyl]-propylcarbamoyl}-heptadecanoic acid tert-butyl ester (5.08 g, 6.00mmol), ethyl-(N’,N’-dimethylamino)propylcarbodiimide hydrochloride (EDC, 1 .73 g, 9.00mmol), N,N-dimethylaminopyridine (DMAP, 0.037 g, 0.30 mmol) and dichloromethane (50mL) were added. The mixture was stirred and diisopropylethylamine (2 mL, 11 .6 mmol) was added in 3 portions. The reaction mixture was stirred for 2 h and the solvents were evaporated. The residue was dissolved in dichloromethane (10 mL) and a solution of hydrochloric acid was added dropwise until pH was lower than 5. The solution was submitted to column chromatography (Silicagel 60, 0.040-0.060 mm; eluent: dichloromethane/methanol 95:5) to provide the product as a yellow oil. Yield: 3.15 g (54 percent).
References
1. Novo Nordisk A/S, Birkebæk JK, Bernt FGM. Dchbs-active esters of peg compounds and their use. WO2018/83335[P], 2018, A, Page column 63; 73; 74.
2. Novo Nordisk A/S, Madsen P, Kjeldsen TB, Hoeg-Jensen T, Jakobsen P, Tagmose TM, Kodra JT. Protease stabilized acylated insulin analogues. US9688737[P], 2017, B2, Page column 103; 104; 106.
3. Janssen Pharmaceutica Nv. Macielag M, Patch RJ, Zhang R, Case MA, Rangwala SM, Leonard JN, Wall M, Chi E. Antibody-coupled cyclic peptide tyrosine tyrosine compounds as modulators of neuropeptide y receptors. US2018/117170[P], 2018, A1, Paragraph 0511.
4. Novo Nordisk A/S; Chen J, Lau JF, Kodra JT, Wieczorek B, Linderoth L, Thøgersen H, Rasmussen SE, Garibay PW. Egf(a) analogues with fatty acid substituents. WO2017/121850[P], 2017, A1, Page column 78; 80.
You may like
Related articles And Qustion
Lastest Price from tBuO-Ste-Glu(AEEA-AEEA-OH)-OtBu manufacturers
US $2.00-0.50/KG2024-04-15
- CAS:
- 1118767-16-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available
US $0.00-0.00/g2024-01-27
- CAS:
- 1118767-16-0
- Min. Order:
- 1g
- Purity:
- >98%
- Supply Ability:
- 15kg per batch