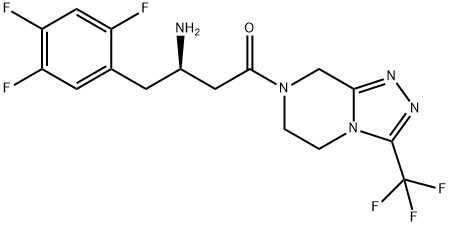
Sitagliptin: Pharmacological Profile and Therapeutic Efficacy in Type 2 Diabetes
General Description
Sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, effectively improves glycaemic control in type 2 diabetes (T2D) patients by increasing active incretin levels. It demonstrates potent and selective inhibition of DPP-4, minimal impact on related enzymes, and neutral effects on body weight and serum lipid parameters. Pharmacokinetic profiles show rapid absorption, proportional AUC increases, and renal elimination. Sitagliptin's therapeutic efficacy as monotherapy or combination therapy is well-established, showing noninferiority to metformin and significant HbA1c improvements. However, its long-term efficacy compared to canagliflozin appears diminished, especially over 52 weeks. Overall, sitagliptin's pharmacodynamics support its role in improving glycaemic control and potentially impacting cardiovascular health in T2D patients, making it an effective option for T2D management.
Figure 1. Tablets of sitagliptin
Mechanism of Action and Effects in Type 2 Diabetes
Sitagliptin, a medication used to improve glycaemic control in patients with T2D, operates through the inhibition of DPP-4, which in turn prevents the inactivation of endogenous incretin hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). By increasing and prolonging active incretin levels, sitagliptin facilitates glucose-dependent increases in insulin release and decreases in glucagon, leading to improved glucose control. The drug exhibits potent and highly selective inhibition of DPP-4, with minimal impact on other related enzymes. Clinical studies have demonstrated that sitagliptin, either as monotherapy or in combination with other antihyperglycaemic drugs, generally enhances b-cell function without marked effects on insulin resistance or sensitivity. Additionally, it tends to have neutral effects on body weight and serum lipid parameters. Notably, in patients with T2D, sitagliptin has shown neutral effects or potential benefits in slowing the progression of carotid intima-media thickness, a surrogate marker of atherosclerotic cardiovascular disease (ASCVD). Furthermore, a thorough QT study in healthy volunteers revealed no clinically meaningful effects on the corrected QT interval at relevant plasma concentrations of sitagliptin. Overall, sitagliptin's pharmacodynamics support its role in improving glycaemic control and potentially impacting cardiovascular health in patients with T2D. 1
Pharmacokinetic
Sitagliptin is a dipeptidyl peptidase-4 inhibitor used in the treatment of type 2 diabetes. The drug shows similar pharmacokinetic profiles in patients with diabetes and healthy volunteers. After oral administration, sitagliptin is rapidly absorbed with peak plasma concentrations observed between 1 to 4 hours. The area under the plasma concentration-time curve (AUC) increases proportionally with the dose of the drug, with an absolute bioavailability of 87%. Most of the administered dose is eliminated unchanged in urine, and metabolism plays a minor role in its elimination. The apparent terminal elimination half-life is 12.4 hours, and renal clearance is about 350 mL/min. No dosage adjustments are required based on age, gender, ethnicity, or BMI. However, patients with moderate to severe renal impairment require dosage adjustment, as plasma AUC levels increase approximately twofold and fourfold, respectively. Sitagliptin has low potential for pharmacokinetic drug interactions and is not a CYP isoenzyme inhibitor or inducer. Potent CYP3A4 inhibitors may alter the pharmacokinetics of sitagliptin in patients with severe renal impairment or end-stage renal disease. Sitagliptin is a p-glycoprotein substrate but does not significantly affect the transport of other drugs. Co-administration of sitagliptin with other drugs does not markedly alter its pharmacokinetics. 2
Efficacy in Type 2 Diabetes Treatment
Sitagliptin, an oral dipeptidyl peptidase-4 (DPP-4) inhibitor, has been extensively evaluated for its efficacy in improving glycaemic control in adult patients with inadequately controlled type 2 diabetes (T2D). In numerous clinical trials, sitagliptin monotherapy demonstrated noninferiority to metformin and significant improvements in HbA1c levels at 24 weeks. When used as initial combination therapy with pioglitazone, sitagliptin provided significantly greater improvements in glycaemic control compared to pioglitazone monotherapy at 24 weeks. Additionally, as an add-on therapy to metformin, sitagliptin was more effective than placebo and noninferior to other oral antidiabetic drugs such as glimepiride, glipizide, and saxagliptin in reducing HbA1c levels. However, after 52 weeks of treatment, add-on sitagliptin showed lower improvements in glycaemic parameters and the proportion of patients achieving a target HbA1c level of less than 7% compared to add-on canagliflozin 300 mg/day, a sodium glucose cotransporter-2 (SGLT-2) inhibitor. The long-term efficacy of sitagliptin in comparison to canagliflozin 100 or 300 mg/day favored canagliflozin, with the latter being superior to sitagliptin in improving HbA1c levels at 52 weeks. Overall, sitagliptin demonstrates efficacy as monotherapy and in combination therapy for T2D, but its efficacy compared to canagliflozin appears to diminish over longer treatment periods. 3
Reference
1. Dhillon S. Sitagliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2010;70(4):489–512.
2. Merck Sharp & Dohme Corp. Januvia? (sitagliptin) tablets: US prescribing information. 2016. https://www.merck.com/. Accessed 26 Sep 2016
3. Scott LJ. Sitagliptin: A Review in Type 2 Diabetes. Drugs. 2017;77(2):209-224.
);You may like
Related articles And Qustion
See also
Lastest Price from Sitagliptin manufacturers

US $200.00-85.00/Kg/Bag2024-05-20
- CAS:
- 486460-32-6
- Min. Order:
- 1Kg/Bag
- Purity:
- 98.5% up
- Supply Ability:
- 20tons

US $18.00-18.00/kg2024-05-10
- CAS:
- 486460-32-6
- Min. Order:
- 1kg
- Purity:
- 99.9
- Supply Ability:
- 5000