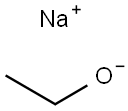
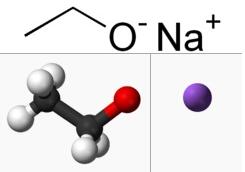
Sodium ethoxide: Application and Preparation
Indication
The sodium ethoxide[1] is a chemical compound with the formula C2H5ONa. It is a white to yellow ish powder that is soluble in polar solvents such as ethanol and methanol. Sodium ethoxide is commonly used as a strong base in organic chemistry reactions, especially in the synthesis of esters from cacboxylic acids and alcohols. It is also used in the production of pharmaceuticals, dyes, and other chemicals.However, it is highly reactive and can be dangerous if not handled properly,as it reacts violently with water and is extremely corrosive to skin and other materials. As a strong alkaline catalyst and ethoxylating agent, sodium ethoxide is widely used in the pharmaceutical and pesticide industries. There are two types of industrial grade sodium ethoxide: liquid and solid. The liquid product is light yellow or brown, and it is an ethanol solution with a content of about 17.5% to 21% of sodium ethoxylate. The solid product is a white or yellowish hygroscopic powder, and the content of sodium ethoxylate is about 95% to 97%. Sodium ethoxide is prone to decomposition and deterioration in the presence of air or water.

Figure 1 Appearence of sodium ethoxide
Application
sodium ethoxide can be used for pharmaceutical products include phenobarbital, butazone, epileptone, methyldopa, tetracaine hydrochloride, ethazine, methotrexate, pyrimidine, and pirofuracampicillin. In addition, it is also used as pesticides and analytical reagents.
The sodium ethoxide can be used as catalyst for extracting flax shive[2]. Their use under ambient conditions to catalyze transesterification reactions is now common in the industrial production of biodiesel. By facilitating the reaction of ester linkages, alkoxide catalysts offer a novel approach for extracting phenolic compounds, while potentially leaving carbohydrate structures intact. The use of ethanol as the solvent for the reaction is also advantageous for environmental reasons. Alkoxide catalysts have already been applied to biomass processing, in analytical methods to quantify acetyl attachments and in modifying natural fibre polymers. Catalyst solution (20 mL) was added to 1 g wb of flax shive in a closed beaker at room temperature (20 ℃), and stirred constantly for a 1h reaction period. The solution was filtered under vacuum and the retained flax shive then rinsed with an additional 100 mL anhydrous ethanol. The filtrate was adjusted to pH < 3 using concentrated HCl, and evaporated under reduced pressure. The residue was dissolved in dichloromethane (50 mL), washed twice with high purity water (30 mL, Millipore Q >18 MΩ resistance), and once using saturated aqueous NaCl solution (30 mL). The organic phase was separated, dried using excess Na2SO4, filtered, and evaporated to dryness under reduced pressure to determine product mass.
Pyrolysis of sodium ethoxide can produce high-quality graphene foams[3]. In a Parr® autoclave reactor (0.5 L volume) were introduced, in a glove bag under argon atmosphere, 6.0 g of sodium , scraped to remove the thin oxide layer, and 15 mL of anhydrous ethanol. A counterpressure of 100 bar was injected in the autoclave, which was then heated at 220 ℃ during 72 h: the internal pressure reaches 195 bar. The as-obtained sodium ethoxide is collected in a glove bag under argon atmosphere.Under argon atmosphere, 5.0 g of solvothermal sodium ethoxide are introduced in an Inconel alloy crucible, which is then placed in a vertical tubular oven under a 500 mL·min−1 nitrogen flow .The oven is heated at around 20 ℃∙min−1 in fixed conditions of temperature (850 ℃) and time (4 h), then allowed to cool down to room temperature, without stopping the nitrogen flow.
Preparation
The second picture shows the chemical reaction equation of sodium ethoxide[4]. The specific preparation steps are as follows: add metallic sodium and inhibitors to the reactor at 0-30 ℃, dropwise add anhydrous ethanol under vacuum conditions of -0.01 to -0.05 MPa, and complete the dropwise addition within 2-4 hours. Continue the reaction at 0-30 ℃ for 2-4 hours to obtain sodium ethoxide, with a sodium ethoxide content greater than 20% and a yield greater than 94%.
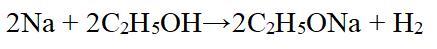
Figure 2 Chemical reaction equation of sodium ethoxide
References
[1]Manoj Kumar Shrivash,Krishna Misra. A Facile Sodium Ethoxide Mediated Knoevenagel Condensation for Production of Ethyl(E)-2-Cyano-3-Substituted Aryl/Alkyl Acrylates[J]. Proceedings of the National Academy of Sciences, India Section A: Physical Sciences,2021.
[2] Robert V. Parsons,Kari A.L. Parsons,John L. Sorensen. Extraction of flax shive using sodium ethoxide catalyst in anhydrous ethanol[J]. Industrial Crops & Products,2011,34(1).
[3] Lucie Speyer,Sébastien Fontana,Sébastien Cahen,Claire Hérold. Simple production of high-quality graphene foams by pyrolysis of sodium ethoxide[J]. Materials Chemistry and Physics,2018,219.
[4] Li HZ, Yu XB, Zhang JJ, et al. A Preparation Method of Sodium Ethanol [P] Shandong Province: CN105294399B, 2018-01-23
);You may like
Related articles And Qustion
See also
Lastest Price from Sodium ethoxide manufacturers

US $0.00/KG2023-09-08
- CAS:
- 141-52-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month

US $0.00/KG2023-09-07
- CAS:
- 141-52-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month