Synthesis and Application of 1-Chloromethyl naphthalene
Physicochemical properties
1-Chloromethyl naphthalene is a prismatic crystal with a density of 1.17 and a melting point of 20-22°C. It has a boiling point of 291-292°C, 167-169°C at 3.33kPa, 150-152°C at 1.73kPa, 135-136°C at 0.8kPa, and a refractive index of 1.6380. It is soluble in benzene and ethanol.
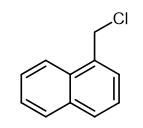
Fig. 1 The structure of 1-Chloromethyl naphthalene.
Synthetic routes
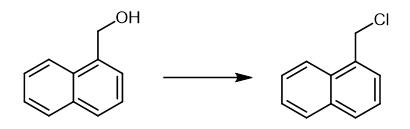
Fig. 2 The synthetic method 1 of 1-Chloromethyl naphthalene.
Dissolve corresponding 1-aryl methanol (28.5 mmol) in dry chloroform (20 mL). Cool the reaction mixture to 5 °C. Add thionyl chloride (42.8 mmol) to the solution at such a rate that the temperature of the reaction mixture do not raise above 10 °C. Allow the temperature of the reaction mixture to raise to room temperature (30 °C). Stir the mixture further for about 30 min. Basify the reaction mixture to pH reaches 8 by slow and careful addition of saturated sodium bicarbonate solution. Extract the mixture with chloroform (3 x 30 mL). Wash the combined organic layer with water (3 x 20 mL). Dry the mixture over anhydrous sodium sulfate. Remove the solvent to obtain 1-chloromethylnapthalene [1].
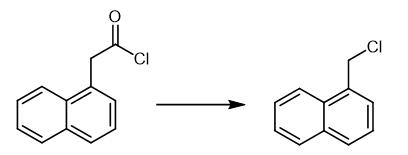
Fig. 3 The synthetic method 2 of 1-Chloromethyl naphthalene.
Weigh 1-naphthylacetyl chloride (102 mg, 0.5 mmol) in a nitrogen-filled glovebox into a 4 mL vial equipped with a10 μm magnetic stir bar. Add pre-mixed solution of Pd[P (o-tol)3]2 (0.1 equiv, 0.05 mmol) and BrettPhos (0.1 equiv, 0.05 mmol) in toluene (1.5 mL). Connect the vial to a reflux condenser, cap with rubber septum and remove from the glovebox. Place an argon balloon on top of the condenser and stir the reaction mixture at 130 oC. Stir for 20 h and cool the reaction mixture to room temperature. Dilute with diethyl ether and filter the resulting solution through a silica plug. Concentrate under reduced pressure and purify the products via flash column chromatography on silica gel (hexanes). 1H NMR (CDCl3, 500 MHz) δ 8.19 (d, J = 8.6 Hz, 1H), 7.93.-.7.87 (multiple peaks, 2H), 7.64 (m, 1H), 7.58.-.7.54 (multiple peaks, 2H), 7.45 (m, 1H), 5.07 (s, 2H); 13C NMR (CDCl3, 125 MHz) δ 134.0, 133.0, 131.1, 129.8, 128.9, 127.7, 126.7, 126.2, 125.3, 123.7, 44.6; HRMS (EI) calcd for C11H9Cl [M]+ 176.0393, found 176.0398 [2].
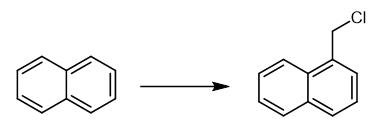
Fig. 4 The synthetic method 3 of 1-Chloromethyl naphthalene.
The reactor was charged with IPB (120.2 g, 1.0 mol) and concentrated aqueous hydrochloric acid solution (300 mL) of surfactant with the desired concentration. The mixture was stirred for 2 h in order to solubilize fully IPB in the surfactant micelle solution (the remaining portion of IPB formed emulsified droplets dispersed in the aqueous phase) and then paraformaldehyde (36.0 g, 1.2 mol) was added as a solid after the reaction temperature reached to 80°C . At the same time, anhydrous HCl gas was bubbled through the reaction mixture with a rate of about 450 mL/min. After the reaction run for a period of time at constant temperature, the reaction was over. After cooling and demulsifying, the organic products were separated and the aqueous solution was extracted with chloroform (3 x 50 mL). The combined organic extracts were neutralized with aqueous saturated sodium bicarbonate solution, washed with water until neutralization and dried over anhydrous sodium sulfate. The analyses by Agilent6890 gas chromatography equipped with a HP-5MS capillary column (30 m x 0.32 mm x 0.25 mm ID) and a hydrogen flame detector were carried out subsequent to filtering of the resulting organic products. The GC conditions were as follows: injection port temperature was set at 280°C and detector temperature at 300°C. Inlet pressures of nitrogen gas and hydrogen gas were 65 and 80 KPa, respectively, and the amount of test specimen was 0.1 ΜL. The temperature program started at 80°C and maintained this temperature for 2 min, then ramped to 165°C at 7°C/min, again up to 250°C at 25°C/min followed by 15 min at 250°C . Finally, the solvent was removed and the residue was distilled under vacuum.Pure chloromethylisopropylbenzene identified by 1H NMR or its authentic sample [3].
Application
As an initiator
The atom transfer radical polymerization of styrene (St) in bulk using 1-(chloromethyl)naphthalene (1-CMN) as an initiator in the presence of CuCl/N,N,N',N'',N''-pentamethyldiethylenetriamine (PMDETA) was investigated at 125 degrees C. The results showed that 1-CMN was an effective initiator with higher initiation efficiency in ATRP of St. The molecular weights of the obtained polystyrene (PS) increased linearly with monomer conversion and the corresponding polydispersity index of polymers remained relatively narrow (1.17-1.30), indicative of well controlled polymerization of St in the presence of 1-CMN/CuCl/PMDETA. The end functionalized PS with naphthalene-label exhibited fluorescence in N,N-dimethylformamide (DMF) [4].
Ligand-Controlled Regioselective Nucleophilic Aromatic Substitution
The palladium-catalyzed reaction of 1-(chloromethyl)naphthalenes 1 with (hetero)arylacetonitriles 2 gives either para- or ortho-acylated naphthalenes (3 or 4) in good to high yields. The regioselectivity can be controlled by the ligand of a palladium catalyst. A sterically bulky ligand, (BuPPh2)-Bu-t, affords para-acylated products 3, whereas a sterically less bulky ligand, Me2PPh, provides ortho-acylated products 4. Further, direct substitution product 5 at the benzylic position is not obtained essentially, although such a reaction at the benzylic position is favorable in ordinary nucleophilic substitutions. In this paper, it was revealed that the benzylpalladium intermediate could react through a different mode (eta(3)-benzylpalladium intermediate or eta(1)-benzylpalladium intermediate) in nucleophilic aromatic substitution. In addition to the interesting mechanistic aspect, the present reaction provides a facile synthetic method for a wide range of diaryl ketones, some of which are not easily available through the previously known procedures [5].
References
[1] DasGupta S, Murumkar P R, Giridhar R, et al. Studies on novel 2-imidazolidinones and tetrahydropyrimidin-2 (1H)-ones as potential TACE inhibitors: design, synthesis, molecular modeling, and preliminary biological evaluation[J]. Bioorganic & medicinal chemistry, 2009, 17(10): 3604-3617.
[2] Malapit C A, Ichiishi N, Sanford M S. Pd-catalyzed decarbonylative cross-couplings of aroyl chlorides[J]. Organic letters, 2017, 19(15): 4142-4145.
[3] Liu Q, Wei W, Lu M, et al. Chloromethylation of aromatic compounds catalyzed by surfactant micelles in oil–water biphasic system[J]. Catalysis letters, 2009, 131(3): 485-493.
[4] Cheng Z, Zhu X, Zhu J, et al. Synthesis of a Well‐Defined Naphthalene‐Labeled Polystyrene via Atom Transfer Radical Polymerization[J]. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, 2005, 42(3): 341-349.
[5] Zhang S, Yamamoto Y, Bao M. Palladium-Catalyzed Ligand-Controlled Regioselective Nucleophilic Aromatic Substitution of 1);
You may like
See also
Lastest Price from 1-Chloromethyl naphthalene manufacturers

US $15.00/kg2024-04-20
- CAS:
- 86-52-2
- Min. Order:
- 1kg
- Purity:
- 99.912%
- Supply Ability:
- 10ton

US $10.00/kg2024-04-19
- CAS:
- 86-52-2
- Min. Order:
- 1kg
- Purity:
- 99.92%
- Supply Ability:
- 50000tons