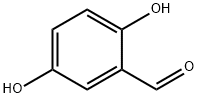
Synthesis and Application of 2,5-Dihydroxybenzaldehyde
2,5-Dihydroxybenzaldehyde (Gentisaldehyde) is a natural antimicrobial agent that inhibits the growth of Mycobacterium avium subsp. paratuberculosis. 2,5-Dihydroxybenzaldehyde also exhibited antibacterial activity against Staphylococcus aureus strains with a MIC50 of 500 mg/L. 2,5-Dihydroxybenzaldehyde is a useful organic synthesis compound that can be used in laboratory research and development processes and in chemical and pharmaceutical synthesis.
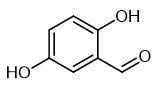
Physicochemical property
2,5-Dihydroxybenzaldehyde is a yellow or khaki crystalline powder with a melting point of 97-99 °C. Its boiling point and density are roughly estimated to be 213.5°C and 1.2667, respectively. It is soluble in water.
Synthetic routes

Fig. 2 The synthetic method 1 of 2,5-Dihydroxybenzaldehyde.
Add 80 mmol of dry paraformaldehyde to a mixture of 12 mmol of the phenol derivative and 2.5 mmol of MgO nanocrystalline (0.1 g). Expose the resulting mixture under microwave irradiation with a power of 650 W. Monitor the progress of the reaction by thin layer chromatography (TLC) developed by n-hexane : ethyl acetate (8 : 2). Add 100 mL of sulfuric acid (15% w/w) to the reaction mixture and heated at 50 °C for 15 min. Cool the reaction mixture to room temperature. Extract the product by dichloromethane (2 x 50 mL). Dry the organic layer over anhydrous magnesium sulphate. Evaporate the solution resulting from filtration to obtain the crude product. Purify the other product by column chromatography using n-hexane : ethyl acetate (95 : 5 to 70 : 30). 1H NMR (400 MHz, CDCl3) /δ (ppm): 10.62 (s, 1H, O-H), 9.82 (s, 1H), 7.10 (dd, 1H,J = 8.8 Hz,J = 2.8 Hz), 7.02 (d, 1H, J= 2.8 Hz), 6.92 (d, 1H,J= 8.8 Hz), 5.15 (s, 1H, O-H); 13C NMR (100 MHz, DMSO) /δ (ppm): 192.17, 154.39, 150.46, 125.02, 122.62, 118.67, 113.56 IR (KBr) /ν (cm-1): 3200-3400 (O-H, phenol), 2877, 2730 (C-H, aldehyde), 1650 (C=O, aldehyde), 1577, 1487 (C=C, Ar), 1284 (C-O) [1].
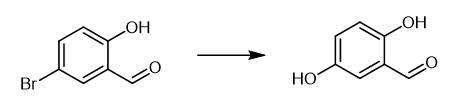
Fig. 3 The synthetic method 2 of 2,5-Dihydroxybenzaldehyde.
Charge an oven-dried Schlenk tube with CuI (4 mg, 0.02 mmol), aryl bromide (1 mmol), 5-bromo-2-(1H-imidazol-2-yl)pyridine (9 mg, 0.04 mmol) and CsOH (504 mg, 3 mmol). Evacuate the tube and backfill with argon. Add DMSO (1 mL), t-BuOH (1 mL) and H2O (0.1 mL). Stir the reaction mixture at 120 °C for 36 h, till the starting material completely converts (monitored by TLC). Acidify the cooled solution mixture to pH = 1-2 with 2 N HCl. Extract the mixture with EtOAc. Wash the combined organic layers with H2O and brine. Dry the combined organic layers over Na2SO4. Remove the solvent in vacuo. Purify the residue with flash chromatography to obtain 2-5-dihydroxybenzaldehyde. 1H NMR (400 MHz, DMSO-d6) δ 10.18 (s, 1H), 10.02 (s, 1H), 9.18 (s, 1H), 6.98 (s, 1H), 6.96 (d, J= 3.2 Hz, 1H), 6.83 (d, J= 8.2 Hz, 1H) Mass Spec MS (EI): 138 (M+) [2].

Fig. 4 The synthetic method 3 of 2,5-Dihydroxybenzaldehyde.
Dissolve benzofuran (0.3 mmol) and [Fe(TF5PP)Cl] (0.33 mol%) in 2 mL of ethanol. Stir the reaction mixture at room temperature (~22 ËC) while protect the mixture from light. Add aqueous hydrogen peroxide (30% w/w) diluted in ethanol (by a factor of 10) to the reaction mixture through a syringe pump for 2 hours. Add total oxidant 4, 6 or 8 mol equivalents relative to the starting material. Perform the reaction as followed by GG-FID analysis to obtain 2,5- dihydroxybenzaldehyde. 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 6.85 (d, 1H, J = 9.1 Hz, H-3), 6.99 (d, 1H, J = 9.1 Hz, H-4), 7.00 (s, 1H, H-6), 9.18 (s broad, 1H, OH-C-5), 10.04 (s broad, 1H, OH-C-2), 10.19 (s, 1H, CHO) 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 112.8 (C-6), 118.3 (C-3), 122.3 (C-1), 124.6 (C-4), 150.1 (C-5), 154.0 (C-2), 191.5 (CHO) [3].
Application
Synthesis of novel spirochromenes
Tandem aldol condensation between steroid sapogenins and hydroxylated benzaldehydes afforded steroidal spirochromenes. Compounds that bear a phenolic hydroxyl group at position C-6', obtained by a reaction with 2,5-dihydroxybenzaldehyde, showed approximately 80% of maximal radical scavenging activity in the 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) assay at 288 nM. In contrast, the starting steroid sapogenins and the spirochromenes without a phenolic group in the side chain proved to be inactive [4].
Syntheses of Violaceoids A and C
The concise syntheses of two alkylated hydroquinone natural products, violaceoids A and C, were accomplished by a protecting-group-free method employing the commercially available 2,5-dihydroxybenzaldehyde as the starting material. The key strategy of the syntheses is the utilization of alkenylboronic acid as both the coupling and temporary protective reagents to efficiently introduce the requisite alkenyl side chain of violaceoid A. Moreover, the synthesis of violaceoid C is reported here for the first time [5].
Facile preparation of some novel Schiff base complexes of uranyl(II), nickel(II), and zinc(II) ions
Synthesis and characterization of some new Schiff base ligands derived from the reaction of 2,5-dihydroxybenzaldehyde with various aliphatic and aromatic diamines and their complexes using UO2(II), Ni(II), and Zn(II) metal ions are reported. The ligands are found to be bound to the metal atom through the oxygen atoms of the hydroxyl groups and nitrogen atoms of imine groups. F [6].
Antimicrobial Activities
Bovine mastitis is a worldwide disease of dairy cattle associated with significant economic losses for the dairy industry. One of the most common pathogens responsible for mastitis is Staphylococcus (S.) aureus. Due to the development and spreading of antibiotic resistance, the search for novel antimicrobial substances against S. aureus is of great importance. The aim of this study was to evaluate two dihydroxybenzaldehydes for the prevention of bovine mastitis. Therefore we determined the minimal inhibitory concentration (MICs) of gentisaldehyde (2,5-dihydroxybenzaldehyde) and 2,3-dihydroxybenzaldehyde of a diverse set of 172 bovine mastitis S. aureus isolates using an automated robot-based microdilution method. To characterize the bovine isolates we determined the genotype by spa-typing, the antimicrobial resistance to eight antibiotic classes using the disk diffusion method and the MICs of three commonly used antiseptics (benzalkonium chloride, chlorhexidine, and iodine). Further we investigated the cytotoxicity of gentisaldehyde and 2,3-dihydroxybenzaldehyde in bovine mammary epithelial MAC-T cells using the XTT assay. The S. aureus strains showed a high genetic diversity with 52 different spa-types, including five novel types. Antibiotic susceptibility testing revealed that 24% of isolates were resistant to one antimicrobial agent and 3% of isolates were multi-resistant. The occurrence of antibiotic resistance strongly correlated with the spa-type. Both dihydroxybenzaldehydes showed antimicrobial activities with a MIC50 of 500 mg/L. The MIC of gentisaldehyde significantly correlated with that of 2,3-dihydroxybenzaldehyde, whereas no correlation was observed with the MIC of the three antiseptics. Cytotoxicity testing using bovine mammary epithelial MAC-T cells revealed that gentisaldehyde and 2,3-dihydroxybenzaldehyde show low toxicity at MIC50 and MIC90 concentrations. In conclusion, gentisaldehyde and 2,3-dihydroxybenzaldehyde exhibited antimicrobial activities against a diverse range of bovine mastitis S. aureus strains at low-cytotoxic concentrations. Therefore, both compounds are potential candidates as antiseptics to prevent bovine mastitis and to reduce the use of antibiotics in dairy cows [7].
[1] Naeimi H, Zakerzadeh E. Efficient microwave-assisted regioselective one pot direct ortho-formylation of phenol derivatives in the presence of nanocrystalline MgO as a solid base catalyst under solvent-free conditions[J]. New Journal of Chemistry, 2018, 42(6): 4590-4595.
[2] Jia J, Jiang C, Zhang X, et al. CuI-catalyzed hydroxylation of aryl bromides under the assistance of 5-bromo-2-(1H-imidazol-2-yl) pyridine and related ligands[J]. Tetrahedron letters, 2011, 52(43): 5593-5595.
[3] Rebelo S L H, Pires S M G, Sim?es M M Q, et al. A green and versatile route to highly functionalized benzofuran derivatives using biomimetic oxygenation[J]. ChemistrySelect, 2018, 3(5): 1392-1403.
[4] Ramos-Enríquez M A, Medina-Campos O N, Pedraza-Chaverri J, et al. Synthesis and radical scavenger properties of novel spirochromenes derived from steroid sapogenins[J]. Steroids, 2015, 98: 132-137.
[5] Narita K, Kimura R, Satoh H, et al. Concise Syntheses of Violaceoids A and C[J]. Chemical and Pharmaceutical Bulletin, 2021, 69(2): 232-235.
[6] Naeimi H, Karshenas A. Facile preparation and characterization of some novel Schiff base complexes of uranyl (II), nickel (II), and zinc (II) ions[J]. Inorganic and Nano-Metal Chemistry, 2017, 47(10): 1480-1487.
[7] Schabauer A, Zutz C, Lung B, et al. Gentisaldehyde and its derivative 2, 3-dihydroxybenzaldehyde show antimicrobial activities against bovine mastitis Staphylococcus aureus[J]. Frontiers in veterinary science, 2018, 5: 148.
);See also
Lastest Price from 2,5-Dihydroxybenzaldehyde manufacturers

US $750.00/KG2024-04-19
- CAS:
- 1194-98-5
- Min. Order:
- 1KG
- Purity:
- 99
- Supply Ability:
- 1000

US $50.00-35.00/kg2024-02-29
- CAS:
- 1194-98-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000kg