Synthesis and Application of Trifluoromethyl iodide
General description
CF3I as a fire extinguishing agent has the characteristics of high fire extinguishing efficiency, good safety performance, high economic utility, no trace after extinguishing, etc. It is the preferred alternative of Bromotrifluoromethane (Halon 1301). As a refrigerant, CF3I is non-combustible, with good oil solubility and material compatibility, and is considered as one of the ideal alternatives to traditional Freon refrigerant components. In addition, CF3I also has a wide range of applications in fluorine-containing intermediates, semiconductor etching, foaming agents and other fields.
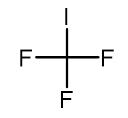
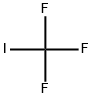
Fig. 1 The structure of Trifluoromethyl iodide.
Synthetic routes
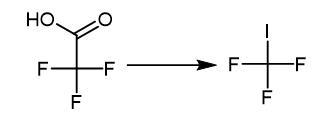
Fig. 2 The synthetic method 1 of Trifluoromethyl iodide.
Introduce 0.1 wt% rhodium/anatase TiO2 (0.016 g, 0.1 mmol) and I2 (0.127 g, 1 mmol) into a Schlenk tube. Fit the tube with a rubber septum. Evacuate and backfill the N2 for three times. Add distilled trifluoroacetic acid (15 mL) to the Schlenk tube through the rubber septum using syringes. Replace the rubber septum by a Teflon cap under N2 flow. Perform the reaction under illumination of 250 W high pressure Hg lamp (365 nm UV) at room temperature for 24 hours [1].
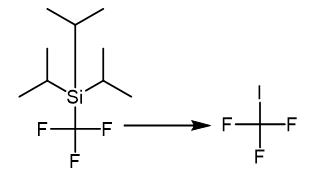
Fig. 3 The synthetic method 2 of Trifluoromethyl iodide.
Add to a 10 mL Schlenk tube charged with a magnetic stir bar t-BuOK (0.1 mmol) and 18 -crown-6 (0.1 mmol). Perform The reaction as described in 5.1 using a solution of iodine (25.4 mg, 0.1 mmol) in freshly distilled THF (0.2 mL) as the electrophile. Seal the tube in the glove box with a septum. Add a solution of iodine (0.1 mmol) in freshly distilled THF (0.016 M, 1.0 mL) to the reaction mixture through a syringe at room temperature. Sonicate the mixture (approximately.Form 1 minutes) until a homogeneous solution. Cool the solution to -78 °C for 10 minutes before the addition of TIPSCF3(10 μL, 0.045 mmol) at -78°C. Maintain the reaction at the same temperature for 10 minutes. Before add a solution of diphenyl disulfide (0.1 mmol) in freshly distilled THF (0.2 mL). Keep the reaction at -78 °C for an additional 60 minutes. Warm the reaction mixture gradually up to room temperature before the determination of the 19F yield with reference to PhCF3 as an internal st and ard [2].
Application
Preparation of trifluoromethylation of iodized aryl compounds catalyzed by copper (I) salt and trifluoromethylzinc reagent
The trifluoromethylation of aryl iodides catalyzed by copper(I) salt with trifluoromethylzinc reagent prepared in situ from trifluoromethyl iodide and Zn dust was accomplished. The catalytic reactions proceeded under mild reaction conditions, providing the corresponding aromatic trifluoromethylated products in moderate to high yields. The advantage of this method is that additives such as metal fluoride (MF), which are indispensable to activate silyl groups for transmetallation in the corresponding reactions catalyzed by copper salt by using the Ruppert-Prakash reagents (CF3SiR3), are not required [3].
As source of trifluoromethyl radicals
A gas-phase kinetic study of hydrogen abstraction from ammonia, methylamine, dimethylamine and trimethylamine has been carried out in a static system by gas chromatography. The photolysis of trifluoromethyl iodide has been used as source of trifluoromethyl radicals. Arrhenius parameters based on Ayscough's value of 10(13.36) cm(3) mol(-1) s(-1) for the recombination of trifluoromethyl radicals have been found. The reactions scheme of trifluoromethyl iodide photolysis in the presence of ammonia and amines has been proposed and kinetically interpreted. The trends in chemical reactivity of CF3 are discussed and interpreted by enthalpy effects [4].
As Detergent
After three fluorine and iodine methane gas liquefaction of various oil have good solution, as a precision instrument cleaning agent, oil cleaning thoroughly, don't need to add surfactant and other additives, cleaning process is a purely physical process, easy cleaning oil and gas separation, after the separation of gas recycling, do not produce secondary pollution.
References
[1] Lin J, Li Z, Kan J, et al. Photo-driven redox-neutral decarboxylative carbon-hydrogen trifluoromethylation of (hetero) arenes with trifluoroacetic acid[J]. Nature communications, 2017, 8(1): 1-7.
[2] Prakash G K S, Wang F, Zhang Z, et al. Long‐lived trifluoromethanide anion: a key intermediate in nucleophilic trifluoromethylations[J]. Angewandte Chemie, 2014, 126(43): 11759-11762.
[3] Nakamura Y, Fujiu M, Murase T, et al. Cu-catalyzed trifluoromethylation of aryl iodides with trifluoromethylzinc reagent prepared in situ from trifluoromethyl iodide[J]. Beilstein Journal of Organic Chemistry, 2013, 9(1): 2404-2409.
[4] Pieni??ek M. Kinetics of the Reaction of Trifluoromethyl Radicals with Nitrogen-Containing Compounds[J]. Polish Journal of Chemistry, 2001, 75(11): 1775-1779.
);You may like
Related articles And Qustion
See also
Lastest Price from Trifluoromethyl iodide manufacturers

US $8.00-1.00/kg2024-04-05
- CAS:
- 2314-97-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $1310.00/KG2023-06-15
- CAS:
- 2314-97-8
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1-10mt