Synthesis and Biological Activity of Tianeptine sodium salt
General description
Tenaneptine sodium salt is a tricyclic antidepressant. Its anti-depression mechanism is different from that of traditional tricyclic antidepressants, which can increase 5-HT reuptake in synaptic cleft, possibly enhancing 5-HT neuron conduction. No affinity for choline or adrenergic receptors. The antidepressant effect was similar to tricyclic, but the tolerance was better than tricyclic.

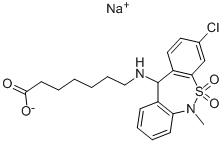
Fig. 1 The structure of Tianeptine sodium salt.
Physicochemical property
Tenaneptine Sodium Salt is a white to tan solid with a melting point of 1800℃. It can be used steadily for one year from the date of purchase. It can be stored for 2 months at -20°C in DMSO or in a solution of distilled water.
Synthetic routes

Fig. 2 The synthetic step 1 of Tianeptine sodium salt.
Charge sodium borohydride (15.04 g) to a solution of 3-chloro-6-methyl-dibenzo[c,f][1,2]thiazepin-11(6H)-one-5,5-dioxide (62.5 g) in methanol (500 ml) at 0-5 °C. Stir the reaction mass for 30 minutes at 0-5 °C, and for 30-60 minutes at room temperature. Filter the solid after the completion of the starting material. Wash the solid with methanol. Dry the solid compound to obtain 3-chloro-6-methyldibenzo[c,f][1,2]thiazepin-11(6H)-ol S,S-dioxide. [1].
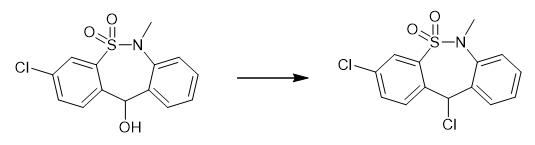
Fig. 3 The synthetic step 2 of Tianeptine sodium salt.
Cool a suspension of 3-chloro-6-methyldibenzo[c,f][1,2]thiazepin-11(6H)-ol S,S-dioxide (58.0 g) in dichloromethane (600 ml) at 0-10 °C. Bubble the HCl gas the through the suspension for 2-4 hour at 0-10 °C. Filter the precipitate after the completion of the starting material. Dry the solid compound to obtain 3,11-dichloro-6,11-dihydro-6-methyl-5,5-dioxodibenzo[c,f][1,2]thiazepine [1].

Fig. 4 The synthetic step 3 of Tianeptine sodium salt.
Charge triethylamine (18.41 g) to 3,11-dichloro-6,11-dihydro-6-methyl-5,5-dioxodibenzo[c,f][1,2]thiazepine (50.0 g) in dichloromethane (400 ml). Stir the contents for 10 minutes. Charge 1-amino,6-cyanohexane (23.0 g) to the reaction over a period of 5-10 minutes. Stir the reaction mixture for 2-6 hours at room temperature. Wash the organic layer with 5% citric acid solution (3×200 ml) until completion of starting material. Wash the organic layer with 200 ml brine solution. Distill the solvent under vacuum to obtain 7-[[(11RS)-3-chloro-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepin-11-yl]amino]heptanenitrile S,S-dioxide [1].

Fig. 5 The synthetic step 4 of Tianeptine sodium salt.
Charge 40% NaOH solution (5.042 g of NaOH, 0.126 mol) to a solution of methanol (300 ml) and tianeptine free acid (50 g) over a period of 10-20 minutes. Heat the contents of the flask to 60-65 °C for 30 minutes. Charge activated carbon (5 g) to the flask. Heat the flask at reflux for 30-45 minutes. Filter the suspension over a Celite pad in hot condition. Distill the methanol. Charge dichloromethane (300 ml) to the flask. Charge sodium sulfate (30 g) and distill dichloromethane. Charge 100 ml acetonitrile to the residue. Cool the reaction mass to -5 to 5 °C. Stir the contents for 2-4 hours. Filter the solid. Dry the compound under vacuum to obtain tianeptine sodium [1].
Biological activity
Antidepressant activity
Recent studies have provided evidence that structural remodeling of certain brain regions is a feature of depressive illness, and the postulated underlying mechanisms contribute to the idea that there is more to antidepressant actions that can be explained exclusively by a monoaminergic hypothesis. Tieneptin (S-1574, [3-chloro-6-methyl-5, 5-dioxy-6, 11-dihydrogenated -(C, F) -dibenzo -(1, 2-thiazine) -11-yl) amino] -7-heptanoate sodium salt) is a compound structurally similar to tricyclic antidepressants whose efficacy and tolerability are well established. The neurobiological properties of tianeptine involve the dynamic interplay between numerous neurotransmitter systems, as well as a critical role of structural and functional plasticity in the brain regions that permit the full expression of emotional learning. Although the story is far from complete, the schema underlying the effect of tianeptine on central plasticity is the most thoroughly studied of any antidepressants. Effects of tianeptine on neuronal excitability, neuroprotection, anxiety, and memory have also been found. Together with clinical data on the efficacy of tianeptine as an antidepressant, these actions offer insights into how compounds like tianeptine may be useful in the treatment of neurobiological features of depressive disorders [2].
The plasticity of excitatory synapses is an essential brain process involved in cognitive functions, and dysfunctions of such adaptations have been linked to psychiatric disorders such as depression. Although the intracellular cascades that are altered in models of depression and stress-related disorders have been under considerable scrutiny, the molecular interplay between antidepressants and glutamatergic signaling remains elusive. Using a combination of electrophysiological and single nanoparticle tracking approaches, Groc et al. report that the cognitive enhancer and antidepressant tianeptine (S 1574, [3-chloro-6-methyl-5, 5-dioxo-6,11-dihydro-(c,f)-dibenzo-(1,2-thiazepine)-11-yl) amino]-7 heptanoic acid, sodium salt) favors synaptic plasticity in hippocampal neurons both under basal conditions and after acute stress. Strikingly, tianeptine rapidly reduces the surface diffusion of AMPA receptor (AMPAR) through a Ca2 t /calmodulin-dependent protein kinase II (CaMKII)-dependent mechanism that enhances the binding of AMPAR auxiliary subunit stargazin with PSD-95. This prevents corticosterone-induced AMPAR surface dispersal and restores long-term potentiation of acutely stressed mice. Collectively, these data provide the first evidence that a therapeutically used drug targets the surface diffusion of AMPAR through a CaMKII--stargazin--PSD-95 pathway, to promote long-term synaptic plasticity [3].
Anticancer activity
Tianeptine sodium salt (TSS) is a selective facilitator of serotonin, but there are no reports regarding anti-invasive effects of TSS. Therefore, we investigated the effect of TSS on the expression of matrix metalloproteinase-9 (MMP-9) and invasion in three different human carcinoma cell lines. Our findings showed that MMP-9 activity was significantly increased in response to tumor necrosis factor-α (TNF-α), and that TSS reduced TNF-α-induced MMP-9 activity in a dose-dependent manner. TSS also downregulated both MMP-9 expression and TNF-α-induced MMP-9 promoter activity. Using a matrigel invasion assay, we showed that TSS significantly attenuated invasive rates in TNF-α-stimulated LNCaP prostate carcinoma cells. Furthermore, TSS suppressed TNF-α-induced NF-B activity, which is a potential transcriptional factor for regulating many invasive genes, including MMP-9, by suppressing IB degradation and nuclear translocation of NF-B subu);
You may like
See also
Lastest Price from Tianeptine sodium salt manufacturers

US $3.20-290.00/g2024-05-01
- CAS:
- 30123-17-2
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $10.00-10.00/ASSAYS2024-04-30
- CAS:
- 30123-17-2
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 tons