Synthesis and Detection method of 3-Bromobiphenyl
General description
3-Bromobiphenyl is an important industrial raw material, but also a very widely used organic synthesis intermediates, it is widely used in pharmaceutical, liquid crystal industry and other fields. In recent years, the export volume of 3-Bromobiphenyl in our country is increasing day by day, the main export object is some liquid crystal display production enterprises in Japan and South Korea.
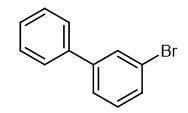
Fig. 1 The structure of 3-Bromobiphenyl.
Physicochemical property
3-Bromobiphenyl is a pale yellow to gray-brown powder with a melting point of 93-94 °C. Its boiling point is 300 °C. It has a density of 1.398 g/mL at 25°C. It is insoluble in water.
Synthetic routes
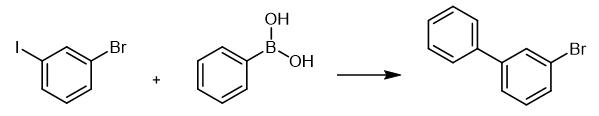
Fig. 2 The synthetic method 1 of 3-Bromobiphenyl.
Add 1-bromo-3-iodobenzene (4.67 g, 16.5mmol), phenylboronic acid (1.83 g, 15.0 mmol), Pd (OAc)2 (101 mg, 0.450 mmol), PPh3 (239 mg, 0.911 mmol), and K2CO3 (6.23 g, 45.1 mmol) to a 200 mL twonecked round-bottom flask. Add toluene (40 mL) and water (40 mL) via syringe and reflux the mixture (bath temp. 100°C) for 24 h. Extract the resulting mixture with ethyl acetate (30 mL x 3). Dry the combined organic layer over Na2SO4 and concentrate in vacuo. Purify the residue to silica gel column chromatography (eluent:hexane). 1H NMR (400 MHz, CDCl3) δ 7.30 (t, J = 7.9 Hz, 1H), 7.35-7.57 (m, 7H), 7.74 (t, J = 1.8 Hz,1H); 13C NMR (100 MHz, CDCl3) δ122.9, 125.8. 127.1, 127.9, 128.9, 130.2, 130.2, 130.3, 139.7,143.4; HRMS (APCI) (M+H+) calcd for C12H9Br: 232.9960, found: 232.9957. [1].

Fig. 3 The synthetic method 2 of 3-Bromobiphenyl.
Carry out all catalytic reactions in reaction vessels open to the air. Charge a round-bottom flask with the newly purchased or freshly recrystallized aryl boronic acid (2.0 mmol), 1,3-dibromobenzene, powdered K3PO4 (2.2 mmol) and toluene (2.5 mL) of technical quality. Stir the mixture vigorously. Heat the mixture to 80 °C for 10 minutes. Add (0.2 mol%) of catalyst by syringe as a 2.5 ml of toluene solution to the mixture. Take the samples periodically from the reaction mixture. Quench the reaction with water. Extract the mixture with ethyl acetate. Analyze the reaction by GC-MS. At the end of catalytic reaction, cool the reaction mixture to room temperature. Quench the mixture with water (adjusted to an appropriate pH when biaryls with acidic or basic groups has to be extracted). Extract the mixture with ethyl acetate (3×40 mL). Dry the combined extracts (MgSO4). Evaporate the combined extracts to dryness. Purify the crude material by flash chromatography on silica gel [2].
Detection method
An electrochemical, signal amplified immunosensor was developed to detect 3-bromobiphenyl (BBP) by using a bio-inspired polydopamine (PDOP)/gold nanocluster (AuNc) as the sensor platform and multienzyme-labeled carbon hollow nanochains as the signal amplifier. The self-polymerized dopamine membrane on the AuNc-modified indium tin oxide (ITO) electrode were characterized by scanning electron microscopy (SEM), atomic force microscopy (AFM), contact angle and electrochemical measurements. Such PDOP/AuNc platform featured the mild cross-linking reaction with the dense immobilization of BBP-antigens (BBP-Ag). Moreover, by using multiple horseradish peroxidase (HRP) and secondary antibodies (Ab2) modified one-dimensional carbon hollow nanochains (CHNc) as the signal enhancer, it held promise for improving the sensitivity and detection limit of the immunoassay. Based on the competitive immunoassay protocol, this immunosensor showed a linear range from 1 pM to 2 nM for BBP with a detection limit of 0.5 pM. Also, it exhibited high sensitivity, wide linear range, acceptable stability and reproducibility on a promising immobilization platform using a novel signal amplifier, which may extend its application in other environmental monitoring [3].
A simple, fast, and sensitive high performance liquid chromatography with electrochemical and UV-Vis detection method for the determination of bromophenols, BPs, (2-bromophenol, 3-bromophenol, and 4-bromophenol), and bromobiphenyls, BBPs, (2-bromobiphenyl, 3-bromobiphenyl and 4-bromobiphenyl) has been developed. The detection limits ranged from 18.2 up to 65.3 mu g/L. The optimized method was successfully applied to river water samples after the development of a simple and fast solid phase extraction (SPE) method allowing the preconcentration and clean up of the analytes. The performance of the complete procedure was satisfactory irrespective of the spiking level with recoveries higher than 65%, and repeatability evaluated as the relative standard deviation, better than 12% [4].
References
[1] Taniguchi T, Nishii Y, Mori T, et al. Synthesis, Structure, and Chiroptical Properties of Indolo‐and Pyridopyrrolo‐Carbazole‐Based C2‐Symmetric Azahelicenes[J]. Chemistry–A European Journal, 2021, 27(26): 7356-7361.
[2] Bolliger J L, Frech C M. Dichloro‐Bis (aminophosphine) Complexes of Palladium: Highly Convenient, Reliable and Extremely Active Suzuki–Miyaura Catalysts with Excellent Functional Group Tolerance[J]. Chemistry–A European Journal, 2010, 16(13): 4075-4081.
[3] Lin M, Liu Y, Chen X, et al. Poly (dopamine) coated gold nanocluster functionalized electrochemical immunosensor for brominated flame retardants using multienzyme-labeling carbon hollow nanochains as signal amplifiers[J]. Biosensors and Bioelectronics, 2013, 45: 82-88.
[4] Quintana M C, Iglesias V, Da Silva M P, et al. HPLC‐UV‐EC Determination of Brominated Organic Compounds in Water[J]. Journal of liquid chromatography & related technologies, 2006, 29(1): 87-98.
);You may like
Lastest Price from 3-Bromobiphenyl manufacturers
US $0.00-0.00/kg2024-04-24
- CAS:
- 2113-57-7
- Min. Order:
- 1kg
- Purity:
- 99.9HPLC
- Supply Ability:
- 20 tons

US $0.00/kg2024-04-12
- CAS:
- 2113-57-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000ton
![140681-55-6 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate); Application; Prevention](/NewsImg/2022-08-03/6379513713249661916550226.jpg)