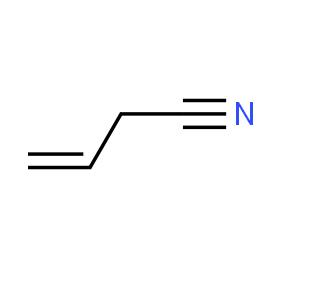
Synthesis and toxicokinetics of Allyl cyanide
Allyl cyanide is an organic compound. Like other small alkyl nitriles, allyl cyanide is colorless and soluble in organic solvents. Allyl cyanide occurs naturally as an antifeedant and is used as a cross-linking agent in some polymers.
Synthesis
Allyl cyanide is obtained by the reaction of allyl acetate with hydrogen cyanide.
A laboratory route to allyl cyanide involves treating allyl bromide with copper(I) cyanide.
CH2=CHCH2Br + CuCN → CH2=CHCH2CN + CuBr
Other allyl halides may be used for this reaction including allyl iodide as done by A. Rinne and B. Tollens in 1871 where iodide is a better leaving group than its bromide equivalent and therefore increases the yield.
Applications
Allyl cyanide may be used as an additive in propylene carbonate-based electrolytes for graphite anodes preventing exfoliation of the anode by film-forming. The underlying mechanism is thought to be a reductive polymerization mechanism.
Toxicokinetics
Allyl cyanide is known to be metabolized in the liver by the Cytochrome P-450 enzyme system (mainly CYP2E1) to cyanide.The absorption and distribution of allyl cyanide in rats is extraordinary fast. The highest concentrations of allyl cyanide were measured in the stomach tissue and stomach contents due to the fact that the stomach is the principal site of absorption after oral administration. The next highest concentration levels were found to be in the bone marrow with a peak in concentration between 0 and 3 hours after administration. The liver, kidneys, spleen and lungs also accumulated allyl cyanide over the course of 48 hours. The highest concentration in the kidney was observed between 3 and 6 hr after dosing. This observation indicates rapid elimination of allyl cyanide. The major route of detoxification is the conversion from cyanide to thiocyanate.[16] Major routes of excretion are through the urine and expired air.
The serotonin and dopamine systems are thought to be involved in the behavioral abnormalities caused by allyl cyanide. Treatment by serotonin and dopamine antagonists caused a reduction in the behavioral abnormalities.Ataxia, trembling, convulsions, diarrhea, salivation, lacrimation and irregular breathing are known effects that are caused by oral ingestion of allyl cyanide.
);You may like
Related articles And Qustion
See also
Lastest Price from 3-Butenenitrile manufacturers

US $15.00-10.00/KG2021-07-02
- CAS:
- 109-75-1
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton