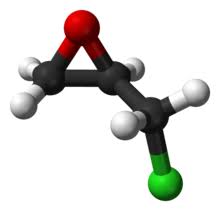
Synthesis and Uses of Epichlorohydrin

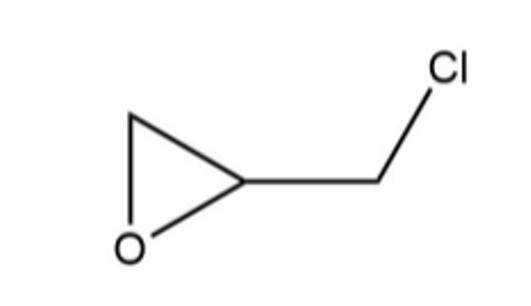
Figure 1 the molecular formula of epichlorohydrin
Uses
Epoxy acrylate (ECH) is one of the basic organic chemical raw materials, mainly used to prepare synthetic glycerin, epoxy resin and chlorohydrin rubber, and can also be used to prepare plasticizers. Surfactants, dyes and pharmaceuticals. Gary L et al. used a case study of epichlorohydrin (EPI) to evaluate the feasibility of extrapolating the dose route of carcinogens at the contact site. By using a dosimetry model to adjust the EPI delivery at the contact site, the EPI cancer efficacy at the contact site was compared in three bioassays involving different dosage routes (gavage, drinking water, inhalation).
The results show that when expressed in external doses, the efficacy of the three routes of administration is quite different (two orders of magnitude). However, when expressed as the peak delivered dose, the inhalation and oral efficacy estimates are similar, and overall, the three efficacy estimates are within 7 times. The results show that the response of the contact site to EPI is more dependent on the rate rather than the delivery route, and the peak concentration is the best way to extrapolate across the dose route.
Synthesis
The high-temperature chlorination method of propylene is mainly composed of three steps of preparing propylene chloride from propylene, synthesizing dichloropropanol (DCP) and preparing ECH. Although this process has been more than 70 years ago and is a classic method for the synthesis of ECH, the yield of this method is low, and the utilization rate of chlorine atoms is only 25%. Even if the selectivity and yield of each reaction reaches 100%, the utilization of chlorine atoms The rate is only 46%.
In addition, the reactants corrode the equipment severely. The production of 1 t of ECH will produce about 40 t of salty wastewater, which will cause serious environmental pollution. At present, the production capacity of domestic production plants using the propylene high-temperature chlorination method accounts for about 40%. The propylene acetate method consists of four steps: propylene to propylene acetate, propylene acetate to hydrolyze propylene alcohol, DCP synthesis and ECH preparation. Compared with propylene high-temperature chlorination, this process has the advantages of low material consumption and high product yield. However, the process flow is long, the acetic acid in the reaction process will cause corrosion to the equipment, and the production of 1 t of ECH will produce about 11 t of wastewater.
At present, this process is basically not used in industrial production. The glycerol method uses glycerol and hydrogen chloride as raw materials to synthesize ECH through two steps of glycerol chlorination and DCP cyclization. The direct epoxidation of allyl chloride is the direct generation of ECH by epoxidation of allyl chloride. This method omits the intermediate steps of preparing DCP from allyl chloride, and the waste water discharge is less than 2% of the propylene method, and it also avoids the formation during the saponification process. Calcium chloride waste residue is a green and clean ECH synthesis process with high atomic economy and has good application prospects. The direct epoxidation of propylene chloride technology to prepare ECH can solve the corrosion and pollution problems in the chlorohydrin and saponification process. When the reaction selectivity is 100%, the atomic utilization rate can reach 84%, and the waste water discharge is only the traditional chlorohydrin process. The COD in wastewater is less than 800 mg/L, and there is almost no discharge of waste residues. It has realized the clean production of ECH. It is a truly green process and it is very hopeful to replace the traditional ECH production process. It has a good development prospect .
The use of hydrogen peroxide as a green oxygen source has been the research hotspot of olefin catalytic epoxidation in recent years. At present, the main catalytic systems are titanium-silica molecular sieve catalytic system and phosphotungstate heteropolyacid catalytic system. The reaction conditions of the titanium-silica molecular sieve catalyst system are mild and the target product selectivity is high. However, the reaction process requires solvents such as methanol/acetonitrile, and the catalyst preparation cost is high, so it needs to be regenerated frequently; An epoxidation reaction occurs with hydrogen peroxide. After the reaction is completed, the hydrogen peroxide is consumed, and the catalyst is separated from the reaction system and can be recycled and reused. However, the amount of chloropropene is large, which increases the subsequent separation cost. The current research and development is mainly reflected in the design of catalysts and improvements in reactors and processes[1].

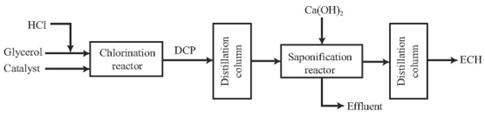
Figure 2 Process flow diagram of ECH production through glycerol chlorination
Reference
[1] Yiyi S, et al. Research progress of green synthesis technology of epichlorohydrin[J].Petrochemical,2021,50(07):719-726.
);You may like
Related articles And Qustion
See also
Lastest Price from Epichlorohydrin manufacturers
US $50.00-1.00/KG2024-03-25
- CAS:
- 106-89-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $3.00/L2024-02-27
- CAS:
- 106-89-8
- Min. Order:
- 1L
- Purity:
- 99.9%
- Supply Ability:
- 3000MT/MONTH