Synthesis of N-Boc-Piperidine-4-carboxylic acid methyl ester
Physicochemical properties
N-Boc-Piperidine-4-carboxylic acid methyl ester is an organic ester and can be used as a pharmaceutical intermediate. Its melting point is 33.0 to 37.0 °C, and its boiling point and density are predicted to be 307.4±35.0 °C and 1.094±0.06 g/cm3, respectively.

Fig. 1 The structure of N-Boc-Piperidine-4-carboxylic acid methyl ester.
Synthetic routes
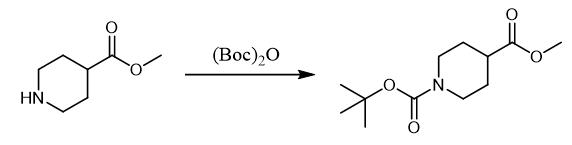
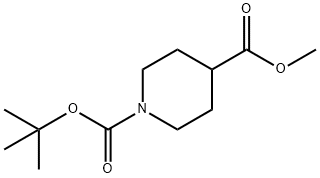
Fig. 2 The synthetic method 1 of N-Boc-Piperidine-4-carboxylic acid methyl ester.
To a stirred suspension of the product of step A (3.7 g, 20.67 mmol) in DCM (75 mL) was added Et3N (14.4 mL, 103.35 mmol) at 0 °C followed by drop wise addition of BOC anhydride (13.3 mL, 62.01 mmol), resulting reaction mixture was stirred at room temperature for 16 hrs. Water (50 mL) was added to the reaction mixture, organic layer was separated and aqueous layer was extracted with dichloromethane. Combined organic layer was washed with brine, dried over sodium sulfate and concentrated under reduced pressure. The crude compound was purified by column chromatography over silica gel (100-200 mesh) using 2% methanol in chloroform as eluent to afford the product (5g, 99%) as a colorless liquid [1].

Fig. 3 The synthetic method 2 of N-Boc-Piperidine-4-carboxylic acid methyl ester.
Add potassium carbonate (1.2 g, 8.7 mmol, 1.0 equivalent) and iodomethane (0.65 mL, 10 mmol) to a solution of 1-(tert-butoxycarbonyl)piperidine-4-carboxylic acid (2.0 g) in DMF (38 mL). Stir the reaction mixture for three hours at room temperature. Pour the reaction mixture into 10% aqueous potassium carbonate (100 mL). Extract the aqueous solution with EtOAc (3 × 50). Wash the combined organic layer with brine. Dry the combined organic layer over Na2SO4. Concentrate the combined organic layer in vacuo. Purify the crude product via column chromatography. 1H NMR (600 MHz, CDCl3): δ 3.99 (s, 2H), 3.67 (s, 3H), 2.82 (t, J = 12.4 Hz, 2H), 2.43 (tt, J = 11.0, 3.9 Hz, 1H), 1.88-1.81 (m, 1H), 1.70-1.55 (m, 2H), 1.44 (s, 9H). 13C NMR (151 MHz, CDCl3): δ 174.9, 154.6, 79.5, 51.7, 41.0, 28.4, 27.9 [3].
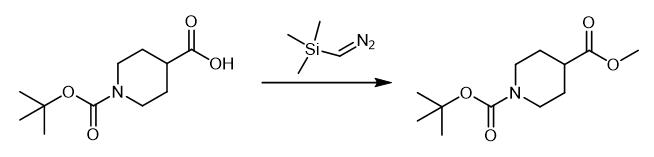
Fig. 4 The synthetic method 3 of N-Boc-Piperidine-4-carboxylic acid methyl ester.
The solution of trimethylsilyl diazomethane in hexanes (2.00 M, 52.9 mL, 106 mmol) was added dropwise to a suspension of 1-tert-butoxycarbonyl-piperidine-4-carboxylic acid (12.14 g, 52.95 mmol) in acetonitrile (100 mL) and methanol (10 mL) at 0 °C. The mixture let stand for 30 minutes and then was stirred at room temperature for 3 hours. The solvent was removed under reduced pressure and product was purified from the residue by column chromatography[n-hex/EtOAc (4.5:1 v/v)] to give 1-tert-butyl 4-methyl piperidine-1,4- dicarboxylate as an oil (11.5 g, 90%). 1H NMR (400 MHz, d6-DMSO) δ 4.02 (dt, 2H, J = 3.5, 13.7 Hz), 3.69 (8, 3H), 2.82 (ddd, 2H, J = 3, 11.5, 14.5 Hz), 2.45 (tt, 1H, J = 3.9, 11.5 Hz), 1.90-1.84 (m, 2H), 1.67-1.57 (m, 2H), 1.45 (8, 9H). MS(ES) m/z 265.8 (MNa+); MS cald: 243.1 (M) [3].
Precautions for the experiment
1. Before the experiment, wear protective glasses, protective clothing, mask, and gloves, and avoid contact with skin.
2. If toxic or irritating substances and harmful substances are encountered during the experiment, the experimental operation should be completed in the glove box when necessary to avoid causing harm to the experimenter.
3. The pipetting nozzle for taking samples should be replaced in time. If necessary, the filter cartridge suction head should be selected as far as possible to avoid cross contamination.
4. When weighing drugs, use weighing paper, take drugs and weigh them in a place without wind to avoid spreading. The container of reagents must be clean and disinfected before use.
5. When taking medicine, try to use multiple medicine spoons separately, clean them after use, dry them, disinfect them and store them.
6. Waste generated after the experiment shall be classified and stored and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
References
[1] Heiser U, Sommer R, Ramsbeck D, et al. Preparation of heterocyclic imidazole derivatives as therapeutic inhibitors of glutaminyl cyclase[P]. PCT Int. Appl., 2011029920, 2011.
[2] Aguilar Troyano F J, Ballaschk F, Jaschinski M, et al. Light‐Mediated Formal Radical Deoxyfluorination of Tertiary Alcohols through Selective Single‐Electron Oxidation with TEDA2+[J]. Chemistry–A European Journal, 2019, 25(62): 14054-14058.
[3] Hirschbein B, Lee C S, Litvak J, et al. Preparation of pyrrolopyridines and analogs as inhibitors of tryptase[P]. PCT Int. Appl., 2006086609, 2006.
);You may like
See also
Lastest Price from N-Boc-Piperidine-4-carboxylic acid methyl ester manufacturers

US $10.00-100.00/kg2024-04-02
- CAS:
- 124443-68-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000 Metric Ton/Month

US $0.00-0.00/kg2023-12-18
- CAS:
- 124443-68-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 50000kg