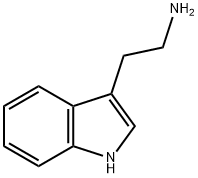
Synthesis, Physicochemical properties and Detection of tryptamine
General description
Tryptamine (Try) is a biological amine formed by the decarboxylic decarboxylic acid of tryptophan in proteins. It is also a neuromodulator or neurotransmitter, which mainly exists in mammals. A precursor (halman alkaloid) formed for Harman. The 5-hydroxyl derivative (5-hydroxytryptamine, 5-HT) is present in mammalian plasma and amphibian skin and has vasoconstrictive effects.

Physicochemical property
Tryptamine is a white needle-like crystal with a melting point of 118℃ (decomposition of 145 ~ 146℃). It is soluble in ethanol and acetone and almost insoluble in ether, benzene, chloroform and water.
Synthetic routes
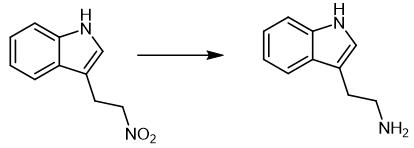
Solubilize 1- (2-nitroethyl) benzene (1 mmol) in solvent (1 mL) in a closed tube and add Pd/C (5 wt%, 50% in water) (0.6 mol%). Add a mixture of sodium hypophosphite monohydrate (4 mmol) and hypophosphorous acid (50% in water) (1 mmol) solubilize in water (2 mL). Stir the reaction mixture for 16 hours at 60 °C. Cool the mixture to room temperature. Dilute the reaction mixture with ethyl acetate (20 mL), basify with a 2.5 M sodium hydroxide solution (10 mL) and filter on Celite®. Extract the aqueous phase with ethyl acetate (3 × 20 mL). Wash the combine organic layers with brine (10 mL) and dry over mag [1].
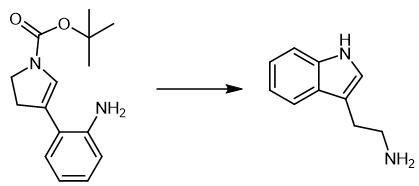
Fig. 3 The synthetic scheme 2 of
Add TFA (2.0 mL) dropwise to a solution of aniline enamine (0.50 mmol) in CH2Cl2 (20 mL) at 0 °C. Warm the resulting solution to room temperature. Stir the resulting solution for 2 hours. Concentrate the reaction mixture under reduced pressure. Dilute the reaction mixture with saturated aqueous NaHCO3 solution until the pH rise above 7. Extract the aqueous layer with CH2Cl2 (3 × 15 mL). Dry the combined organic layers over Na2SO4. Concentrate the combined organic layers under reduced pressure to afford tryptamine as a white solid (78.5 mg, 98 %) [2].
Pharmacological action
Chronic cluster headache
Plasma levels of tyramine, tryptamine, serotonin, 5-hydroxyindolacetic acid, noradrenalin, adrenalin and the markers of arginine metabolism such as arginine, homoarginine, citrulline, NG,NG-asymmetric dimethyl-L-arginine (ADMA) and NG -monomethyl-L-arginine (NMMA), were measured in 23 chronic cluster headache patients (10 chronic cluster ab initio and 13 transformed from episodic cluster) and 28 control subjects. The plasma levels of tyramine, tryptamine, noradrenalin and adrenalin were found several times higher in chronic cluster headache patients compared to controls, whereas the plasma levels of arginine, homoarginine and citrulline were significantly lower. No differences were found in the plasma levels of serotonin, 5-hydroxyindolacetic, ADMA and NMMA between chronic cluster headache patients and control subjects. These results provide support for a role of tryptamine in the pathogenesis of chronic cluster headache and, in particular, in the duration of the cluster bouts. In addition, the low levels of the nitric oxide substrates together with the high levels of noradrenalin and adrenalin suggest an activation of endothelial trace amine-associated (TAAR1) receptors followed by the release of nitric oxide in the circulation that may constitute the final step of the physiopathology of cluster crisis [3].
A novel electrochemical sensor was designed and successfully constructed by the molecularly imprinting technology for the efficient detection of tryptamine. The sensor was modified with the composite film of multiwalled carbon nanotubes-ionic liquid and tryptamine imprinted chitosan-porous Pt. The morphology and structure of the nanocomposite were characterized by scanning electron microscope, energy dispersive spectrometer, and X-ray diffraction. Results confirmed the successful construction of the target electrochemical sensor. The performance of the as-prepared sensor was characterized by the electrochemical methods. Under the optimal conditions, the ideal linear range of the electrochemical sensor toward tryptamine was from 5.0 × 10–8 to 6.0 ×10–5 M with the excellent limit of detection of 4.58 × 10–8 M at S/N = 3. The as-prepared electrochemical sensor showed good stability, reproducibility and selectivity for tryptamine, which is very important for its practical usage. Ultimately, the sensor was successfully applied to detect tryptamine in food samples of both lactobacillus beverage and cheese [4].
References
[1] Letort S, Lejeune M, Kardos N, et al. New insights into the catalytic reduction of aliphatic nitro compounds with hypophosphites under ultrasonic irradiation[J]. Green Chemistry, 2017, 19(19): 4583-4590.
[2] Nicolaou K C, Krasovskiy A, Majumder U, et al. New synthetic technologies for the construction of heterocycles and tryptamines[J]. Journal of the American Chemical Society, 2009, 131(10): 3690-3699.
[3] D’Andrea G, Bussone G, Di Fiore P, et al. Pathogenesis of chronic cluster headache and bouts: role of tryptamine, arginine metabolism and α1-agonists[J]. Neurological Sciences, 2017, 38(1): 37-43.
[4] Guo W, Xia T, Zhang H, et al. A molecularly imprinting electrosensor based on the novel nanocomposite for the detection of tryptamine[J]. Science of Advanced Materials, 2018, 10(12): 1805-1812.
);You may like
See also
Lastest Price from Tryptamine manufacturers

US $10.00/ASSAYS2024-05-02
- CAS:
- 61-54-1
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton

US $0.00/G2024-05-02
- CAS:
- 61-54-1
- Min. Order:
- 1G
- Purity:
- 99%
- Supply Ability:
- 20