Synthetic method of sulfanilic acid
Background
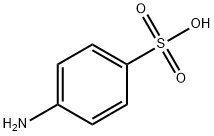
Sulfanilic acid, also known as p-aminobenzenesulfonamide, with the molecular formula C6H8N2O2S, is an organic compound with medicinal value[1]. It is commonly used in the pharmaceutical industry and is the main raw material for the synthesis of sulfonamides. The melting point of sulfanilic acid is 164-166°C, the boiling point is 400.5°C, and the flash point is 196.0°C, the density is 1.08g/cm3, and the refractive index is 1.628. The appearance of sulfanilic acid is white to pale yellow crystalline powder. Sulfanilic acid is slightly soluble in cold water, ethanol, methanol, ether and acetone, easily soluble in boiling water, glycerol, hydrochloric acid, potassium hydroxide and sodium hydroxide solution, insoluble in chloroform, ether, benzene, petroleum ether.

Picture 1 The physical map of sulfanilic acid
The properties of sulfanilic acid
Sulfanilic acid is acid-base amphoteric, soluble in acid or alkali solution, and its sodium salt is often formulated for injection[2]. Sulfanilic acid has an aromatic primary amino group in a reducing molecule, which is easy to be oxidized. Under the catalysis of sunlight and heavy metals, the oxidation is accelerated, especially the sodium salt is more easily oxidized under alkaline conditions, and the color can gradually become darker when exposed to light. Keep tightly closed. The oxidation products are mainly azo compounds and azo compounds (orange-yellow). The hydrogen atom on the sulfonamide group of sulfanilic acid can be replaced by metal ions (silver, copper, cobalt) in the copper salt-forming reaction, resulting in the formation of insoluble metal salt precipitates of different colors, which can identify and distinguish different sulfonamide drugs. In the diazotization and coupling reaction of primary aryl amino groups in acidic solution, sulfanilic acid can react with sodium nitrite to undergo diazotization reaction, and the content of sulfonamides can be determined by using this property. The resulting diazonium salt is coupled with β-naiol under alkaline conditions to generate a scarlet azo compound, which can be used for identification. In addition, sulfanilic acid can also undergo bromination reaction, condensation reaction with aromatic aldehyde, etc. In addition, sulfanilic acid can also undergo bromination reaction, condensation reaction with aromatic aldehyde, etc.
The synthetic method of sulfanilic acid
Sulfanilic acid can be used for the determination of nitrite[3]. Biochemical Research. Organic Synthesis. Sulfanilic acid is an important intermediate of sulfonamide drugs. This product has strong antibacterial effect on hemolytic streptococcus, meningitis and coccus, but due to poor efficacy and high toxicity, it is rarely used for oral administration. It is also rarely used. It is used as an intermediate for the synthesis of other sulfonamides, and is also used as a raw material for the synthesis of agricultural "Huangcaoling" abroad. Sulfanilic acid can also be used as an analytical reagent, such as a reagent for the photometric determination of nitrite and sodium nitroferricyanide. For biochemical research, organic synthesis and pharmaceutical industry. Sulfanilic acid is the main raw material for synthesizing sulfonamides. In addition to preparing crystalline sulfonamides for external anti-inflammatory, it can also synthesize other sulfonamides such as sulfamidine, sulfamethoxazine, and sulfamethazine.
Precautions
When transporting, store and transport according to general chemical regulations. To ensure that the packaging of sulfanilic acid is complete, and to handle sulfanilic acid lightly; keep the warehouse ventilated, away from open flames, high temperatures, and store it separately from oxidants. When storing, ensure that sulfanilic acid is stored in a cool, ventilated, dry place, protected from heat, sun, and moisture. Storage and transportation according to general chemical regulations. It can be packed in a sack lined with a plastic bag and a sack, or a plastic bag with an inner layer, a kraft paper bag for the middle layer, and a fiber cloth bag for the outer layer. Generally 40kg per bag.
Reference
1 Lin Haidan, Xie Shouxin, Feng Dexiong, etc. Determination of sulfonamide drug residues in animal-derived food by solid-phase extraction-high performance liquid chromatography. 2003
2 Li Yanwen, Mo Qihui, Zhao Na, etc. Determination of sulfonamide antibiotics in water and soil by high performance liquid chromatography. 2008
3 Pang Guofang, Cao Yanzhong, Zhang Jinjie, etc. Simultaneous determination of 16 sulfonamide residues in poultry tissues by liquid chromatography-tandem mass spectrometry. "Analytical Chemistry", 2005
);You may like
See also
Lastest Price from Sulfanilic acid manufacturers

US $1.00/kg2024-02-27
- CAS:
- 121-57-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 50000tons

US $1.00/kg2023-12-11
- CAS:
- 121-57-3
- Min. Order:
- 1kg
- Purity:
- 99.00%
- Supply Ability:
- 20tons