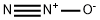Systemic effects of Nitrous oxide
Nitrous oxide is an inorganic gas first synthesised by Joseph Priestley in 1772. Despite early knowledge of its analgesic and anaesthetic properties, however, it was not introduced into mainstream medical/dental practice until the late 1800s.
Uses
Adjuvant to the induction and maintenance of general anaesthesia. 2. Analgesic agent, especially as Entonox (see later). 3. A refrigerant in cryosurgery.
Presentation and storage
Nitrous oxide is presented as a liquid in French-blue cylinders at a pressure of 44 bar. The gauge pressure, however, bears no correlation with the cylinder content until all the nitrous oxide is in the gaseous phase. The volume of liquid nitrous oxide in a cylinder is determined by the ‘filling ratio’. This is the ratio of the mass of nitrous oxide in the cylinder to the mass of water that the cylinder could hold if it were full. I n temperate regions (such as the UK) the filling ratio is 0.75; in warmer climates it is reduced to 0.67 to avoid cylinder explosions. Cylinders should be kept upright and undergo regular testing, the details of which should be recorded on the plastic disc between the cylinder valve and neck and engraved onto the cylinder body. It is also presented as piped medical gas and vacuum (PMGV) at 4 bar.
Physical properties
Nitrous oxide is a sweet-smelling, non-irritating, colourless gas with the following properties:
• Molecular weight 44 Da
• Boiling point –88°C
• Critical temperature (the temperature above which a gas cannot be liquefied however much pressure is applied) 36.5°C
• Critical pressure (the minimum pressure that causes liquefaction of a gas at its critical temperature) 72.6 bar
Nitrous oxide is not flammable but it supports combustion of fuels in the absence of oxygen.
Mechanism of action
Nitrous oxide appears to exert its activity at different types of receptors. It has an inhibitory action on NMDA glutamate receptors and stimulatory activity at dopamine, α1 - and α2 -adrenergic and opioid receptors. The analgesic action of nitrous oxide is probably mediated by activation of opioid receptors in the periaqueductal area of the midbrain. This leads to modulation of nociceptive pathways through the release of noradrenaline and activation of the α2 -adrenoreceptors in the dorsal horn of the spinal cord. Because of its analgesic properties, nitrous oxide is usedin combination with volatile agents as part of a general anaesthetic, which reduces the MAC of volatile anaesthetic agent required.
Systemic effects
RS:
• Decreases tidal volume but increases respiratory rate and hence maintains minute ventilation.
• Reduces the ventilatory response to hypoxaemia and hypercapnia.
• Depresses tracheal mucociliary flow and neutrophil chemotaxis and may increase the incidence of postoperative respiratory complications.
• Non-irritant and does not cause bronchospasm.
• Can cause diffusion hypoxia (see next section).
Pharmacokinetics
Nitrous oxide is often said to be a good analgesic but a weak anaesthetic. The latter refers to the fact that its MAC value is 105%. This value was calculated theoretically from its low oil/water solubility coefficient of 3.2 and has been confirmed experimentally in volunteers anaesthetised in a pressure chamber compressed to 2 atmospheres. As it is essential to administer a minimum FIO2 0.3 during anaesthesia, nitrous oxide alone is insufficient to produce an adequate depth of anaesthesia. Therefore nitrous oxide is usually administered in combination with a volatile agent.
);