Tellurium Crystal
Te is important as the thermocouple material. Te2 is obtained at 1400℃–1800℃. Te burns to form TeO2 at high temperatures in air, emitting a bluish white flame. Te reacts intensely with halogen.
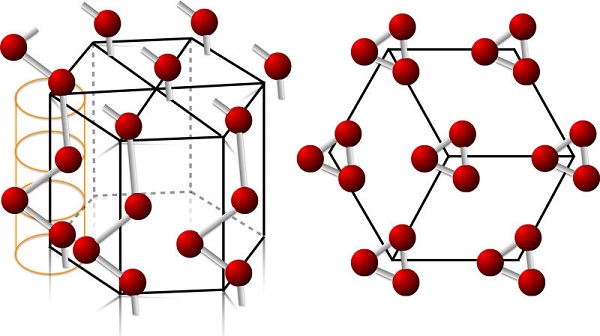
Crystal System
The space lattice of Te belongs to the hexagonal system D43 or D63, with the lattice constants of a=0.4447 nm, c=0.5915 nm (20℃), and the bond angle of 1028. Te forms the chain-like lattice arranged helically around the c axis and the spacing between adjacent Te along the chain is Te– Te=0.286 nm.
Production
A single crystal is difficult to grow and polycrystals are usually obtained from the melt solution. Single crystals of about 2×2×20 mm3are grown by the vapor phase growth. The cleavage plane is parallel to the c axis and to make cleavage or to cut to the other direction is difficult. The electrical conducting layer is formed at the surface by rubbing and it needs to be removed by using corrosives. It becomes p-type by doping As, Sb, Bi, Br, I, etc.
Thin films are deposited by vacuum evaporation using a conical basket heater of Mo, Nb, Ni, Fe or chromel, or an alumina crucible with some external heater. It is very easy to evaporate and it gives contamination to the vacuum chamber. It is important to clean fully after vacuum deposition).
);You may like
See also
Lastest Price from Tellurium manufacturers

US $0.00-0.00/KG2024-02-01
- CAS:
- 13494-80-9
- Min. Order:
- 10mg
- Purity:
- 99%
- Supply Ability:
- 2000tons

US $1.00/g2019-07-06
- CAS:
- 13494-80-9
- Min. Order:
- 50 g
- Purity:
- 99.9%
- Supply Ability:
- 20kg