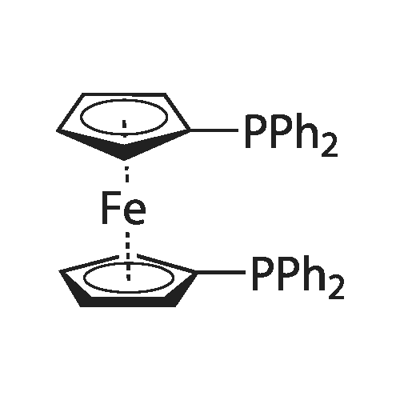
The catalytic property of 1,1'-Bis(diphenylphosphino)ferrocene
Introduction
The bidentate ligand 1,1'-bis(diphenylphosphino)ferrocene (dppf) is a widely used component in catalytic systems and its role in this capacity has been expertly reviewed elsewhere. The focus of this Perspective is the increasing use of dppf in the synthesis and matrix of 21st century materials. The ferrocene core imparts fine control to catalytic C–C and C–X coupling reactions used to manufacture a range of functional macromolecules from tailored dyes and OLED components to precisely engineered conducting polymers and thermoplastics. This ligand’s limited flexibility resembles a ball and socket joint with simultaneous rotation and constrained perpendicular freedom. This uniquely restricted range of movement stabilizes a diverse array of ground and transition states for these important transition metal catalysed coupling reactions. It may also contribute desirable mechanical or electronic functionality as a bridging or chelating component in a coordination array, metallocycle or larger supramolecular assembly. The ferrocene offers steric bulk and crystallinity to these materials aiding chemical stability and ease of handing. It’s oxidizability assists characterization and may be tailored to provide or complement photoor electroactivity. Dppf containing materials have been designed with diverse functions from cooperative luminescence to host–guest complexation. It is likely that this ubiquitous lab companion will increasingly find its way into the fabric or processing of future functional molecular materials.
Catalysis of Dppf
Ferrocenyl diphosphines, most commonly 1,1′-bis(diphenylphosphino)ferrocene (dppf), are among the most effective ligands for an astonishingly wide variety of transition metal catalysed transformations. The role of dppf in catalysis has been comprehensively reviewed,1,2 but the increasing use of this stable, redox-active, conformationally flexible diphosphine in materials science, both as a reagent and as a component, is worthy of contemplation. The popularity of dppf stems from (i) its ease of synthesis from inexpensive ferrocene; (ii) air, moisture and thermal stability; (iii) ease of handling; (iv) solubility in common solvents; (v) conformational flexibility; (vi) the stability and comparative ease of isolation of resulting Group 6–12 metal complexes; (vii) easy oxidation of the ferrocene moiety; (viii) ESI-MS, NMR and XRD-friendly functionality and, most importantly, (ix) the comparatively high success rate among the common phosphines and diphosphines in catalysis. The ferrocenyl backbone gives a large natural bite angle to dppf as a diphosphine. The computationally calculated preferred P–M–P bite angle for dppf is 95.60° compared to an average P–M–P angle of 98.74° determined from X-ray crystal structures.3 However, if a bidentate ligand is to stabilize intermediates of different geometry and electron density [e.g. from Pd(0) to Pd(II)] during oxidative addition and reductive elimination, then a low energy barrier to the large expansion or contraction of the P–M–P angle is clearly an advantage. The ferrocene backbone offers the flexibility to accommodate open trigonal planar geometries as well as more compressed square planar, trigonal bipyramidal and octahedral intermediate complexes, with a minimal energy penalty. This versatility results in both the stabilization of a wide range of catalytically active transition states and also a plethora of metal–dppf materials with nuclearity ranging from unity to essentially infinity.
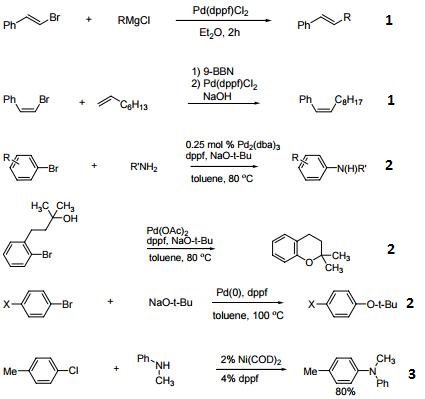
Dppf Catalysis Example
Dppf figures prominently in catalytic C–B, C–C, C–N, C–O, C–P and C–halide coupling reactions with metals including Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Au and Zn. Modern catalysis studies of this type usually report a comparison of results with different mono- and bidentate phosphine ligands and dppf is almost invariably included. It is quite often (but not always) the top performer. Some interesting, recent examples involving the synthesis of small molecules are given in Picture 1.
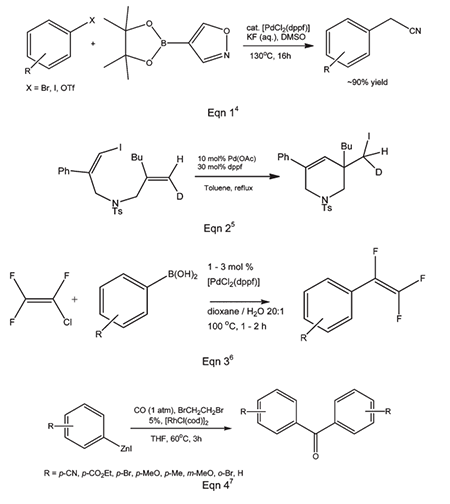
Picture 1 The synthesis of small molecules
Reference
1 Bandoli G, Dolmella A. Ligating ability of 1, 1′-bis (diphenylphosphino) ferrocene: a structural survey (1994–1998)[J]. Coordination Chemistry Reviews, 2000, 209(1): 161-196.
2 Young D J, Chien S W, Hor T S A. 1, 1’-Bis (diphenylphosphino) ferrocene in functional molecular materials[J]. Dalton Transactions, 2012, 41(41): 12655-12665.
You may like
Related articles And Qustion
Lastest Price from 1,1'-Bis(diphenylphosphino)ferrocene manufacturers

US $5.00-2.00/kg2024-04-16
- CAS:
- 12150-46-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available
US $35.00-286.00/g2024-02-23
- CAS:
- 12150-46-8
- Min. Order:
- 100g
- Purity:
- 0.98
- Supply Ability:
- 100kg