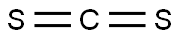The Hybridization and Polarity of Carbon Disulfide
Carbon disulfide exists in the liquid state at standard conditions of temperature and pressure. It smells sweet like ether. This compound is considered as the building block in the world of organic chemistry. It is widely used in the industries as a non-polar solvent.
Hybridization
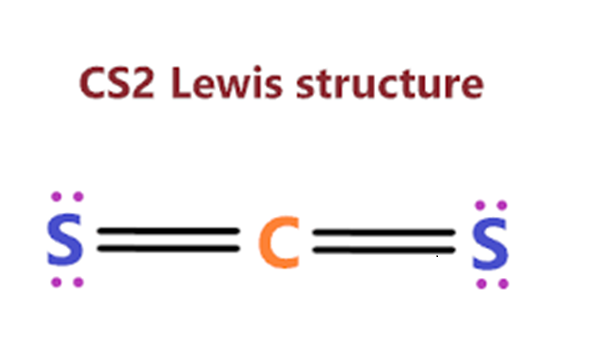
It is essential to know the type of bonding in the molecule to understand its hybridization. In CS2 molecule, two double bonds are formed consisting of eight valence electrons. Thus it takes up eight valence electrons out of 16 valence electrons. These valence electrons that form the double bond with the Carbon atom are in 2s and 2p orbital of the Carbon atom. These orbitals then combine, creating the hybrid of sp orbitals.
This hybridization is known as sp hybridization. These two hybrid orbitals form sigma bonds with Carbon. Remaining eight valence electrons are taken up by the two unused orbitals of p. These electrons form the pi bonds with sulfur and are shown as the lone pairs on the sulfur atoms.
The steric number is the sum of the number of atoms bonded on the central atom and the number of lone pairs of electrons attached to the central atom.
The formula to find the steric number for any molecule is:
Steric Number (SN) = No of sigma bonds on the central atom +No of pi lone pairs on the central atom
Here in the CS2 molecule, the number of sigma bonds on the central atom is two, and there are no lone pairs on the central atom as its octet is complete by sharing the valence electrons.
Thus SN of CS2 molecule = 2+0=2
Steric Number of CS2 is 2; thus its hybridization has two hybrid orbitals making it an sp hybridization.
Polarity
Carbon disulfide (CS2) has a carbon (C) atom surrounded by two sulfur (S) atoms. Carbon has four valence electrons, and sulfur has six. Each sulfur atom shares a double bond with the central carbon atom, completing the octets of all three atoms. Carbon does not have any lone electron pair. Therefore, the molecular structure of CS2 is linear.

The electronegativity of carbon is 2.55, and that of sulfur is 2.58. The electronegativity difference is 0.03. Therefore, the C-S bond is slightly polar. The directions of the two dipole moment vectors are equal and opposite. As a result, they cancel, making CS2 nonpolar.
);You may like
Related articles And Qustion
See also
Lastest Price from Carbon disulfide manufacturers

US $30.00/kg2024-02-27
- CAS:
- 75-15-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $15.00-10.00/KG2021-07-13
- CAS:
- 75-15-0
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton