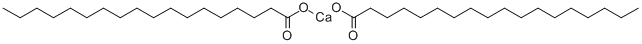The preparation of calcium stearate
Introduction
Calcium stearate n. (C17H35COO)2. (1) A non-toxic stabilizer and lubricant. It is not often used alone because of its early color development, but is used in combination with zinc and magnesium derivatives and epoxides in manufacturing other non-toxic stabilizers. (2) Calcium salt of stearic acid. A metallic soap used in paints as wetting aid for pigments, flatting agents, and additive for improving sanding sealers[1]. Calcium stearate, an internal lubricant which reduces friction between PVC grains during processing, is commonly used in plasticized PVC which is melt processed External lubricants, which are essential in rigid PVC compounds, are not always necessary when plasticizers are present.

Picture 1 Calcium stearate powders
Calcium stearate production method
The invention discloses a calcium stearate production method[2]. The calcium stearate production method is characterized by including steps of firstly, subjecting ammonium stearate and calcium hydroxide to reaction to generate calcium stearate and ammonia water; secondly, when calcium hydroxide turbid liquid is insufficiently added, stearic acid is excessive, subjecting the stearic acid and the ammonia water to reaction to generate the ammonium stearate, and preparing the ammonium stearate required in the first step; and when calcium hydroxide turbid liquid is slightly excessively added, subjecting the ammonium stearate and the calcium hydroxide to reaction and generating calcium stearate and ammonia water, wherein the ammonia water is freed and is decomposed to generate ammonia gas on the condition of the pH (potential of hydrogen) value of 9-9.5 of the reaction system at the temperature of 65-85 DEG C, and filtrate is composed of water and precious little calcium hydroxide soluble in water. The calcium stearate production method is simple in process and is low in reaction temperature and energy consumption and low in requirements for equipment as compared with a double decomposition process, no reflux reaction is needed in the later stage of reaction, even general double decomposition process production equipment can produce products, economic benefits are evident, and accordingly, the calcium stearate production method is applicable to further popularization.
Calcium stearate preparation technology
The invention discloses a calcium stearate preparation technology[3]. The technology comprises the following steps: 1, adding 40-45 parts ofstearic acid, 10-15 parts of calcium hydroxide, 1-5 parts of zinc oxide and 20-30 parts of distilled water into a reaction kettle, and uniformly stirring; 2, heating the reaction kettle to 90DEG C while starting a vacuum system; 3, sampling, analyzing free acids and calcium hydroxide, and ending the reaction if continuous three-time detection results show that the content of calcium hydroxide is zero and the content of the free acids is smaller than 0.5%; and 4, separating the obtained product from raw materials, and drying the product until the water content is smaller than 2% in order to obtain the calcium stearate product. The product prepared in the invention has the advantages of large density, reduced fluidity, unlikely suspension in air, and reduction of the dust pollution.
Calcium stearate dye for pvc
Process consists of placing a powdered dye and calcium stearate (natural) in a container and then grinding continuously with warming at a temp. sufficient to soften the Ca stearate[4]
Calcium stearate: a green corrosion inhibitor for steel in concrete environment
Among the available methods to prevent corrosion, the use of corrosion inhibitors is most attractive from the view point of economy and ease of application[5]. There are many investigations on use of inhibitors for corrosion of steel. Most of the commercial inhibitors are either toxic or show adverse effect on concrete properties. Further the environmental legislations have restricted the use of many inhibitors. In view of this development of environmentally benign green inhibitors has become common practice in recent years. In continuation of our work on Green Inhibitors in the present work we have synthesized calcium stearate corrosion inhibitor and investigated its inhibiting action on corrosion of steel. It is a non-toxic inhibitor.
Reference
1 Calcium stearate. In: Gooch, J.W. (eds) Encyclopedic Dictionary of Polymers. Springer, New York, NY, 2007.
2 MAO SHENGHUA; ZHOU SONGTAO; MAO YINGYING, CN102875356A
3 WANG MINGFU, CN103880632A.
4 specif. 150 degrees C for about 24 hours) and then allowing to cool.(EBSA S A [CH] CH500273A;FR1596344A.
5 M.A. Quraishi, Calcium Stearate: A Green Corrosion Inhibitor for Steel in Concrete Environment, J. Mater. Environ. Sci. 2 (4) (2011) 365-372.
);You may like
See also
Lastest Price from Calcium stearate manufacturers

US $0.00/kg2024-04-12
- CAS:
- 1592-23-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $100.00/kg2024-02-27
- CAS:
- 1592-23-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100 tons