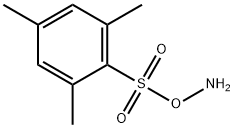
The synthesis method and uses of O-Mesitylenesulfonylhydroxylamine
Description
O-Mesitylenesulfonylhydroxylamine (MHS) is a useful organic compound. This compound is a Pale Yellow to Pale Beige oil with the chemical compound C9H13NO3S. MSH is slightly soluble in DMSO. This molecule contains a benzene ring and an amine group. The functional group makes it special in organic synthesis.
Synthesis method
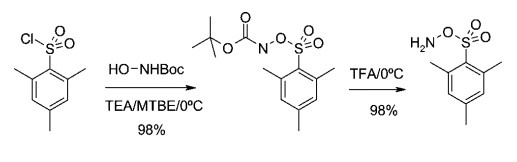
P-Trifluoroacetic acid (5 L) was cooled to 2 °C. Carbamic acid, N-BocO- mesitylenesulfonylhydroxylamine (1695 g, 5.37 mol) was added portion-wise over 1 h. The reaction was stirred at 2 °C for 90 min and monitored by TLC (n-hexane/ethyl acetate 8:2). After 90 min, TLC showed completed conversion. Crushed ice was added (∼2 L) followed by water (4 L), increasing the internal temperature to 2 °C. A white solid appeared when the internal temperature increased to 8 °C. Additional ice/cold water was added (6 L). After 15 min, the solid was filtered, washed with water (20 L) until the pH ≈ 7, and dried of excess water for 15 min. O-Mesitylenesulfonylhydroxylamine (MSH, 9) (1747 g, 151,00% yield) was isolated as a white solid. This material contained 33% of water, and the material was stored in plastic bottles in the freezer for 2 h[1].
Uses
O-Mesitylenesulfonylhydroxylamine is an oxidizing and aminating reagent with great potential to oxidatively eliminate the cysteine thiol modification to dehydroalanine. MSH results in the regeneration of dehydroalanine, allowing a "functional switch" by the subsequent addition of a different thiol. The Davis research group used MSH oxidation to eliminate the thioether bond in the serine protease mutant S-ethylcysteine under a mild system of pH=8 and successfully converted it into dehydroalanine[2]. Subsequently, the MSH oxidative elimination method was successfully applied to the cleavage of thioether bonds in cysteine sulfhydryl-microcystin modifications.
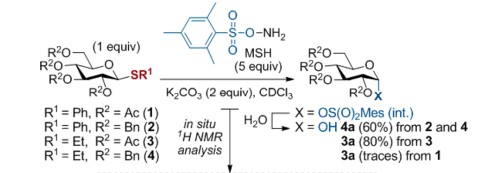
O-Mesitylenesulfonylhydroxylamine could be used to synthesize many organic compounds. The N+-thiophilic reagent MSH could selectively activate thioglycosides as a promoter. MSH reactivity with sulfur species proceeds via direct S-to-N nucleophilic attack and typically affords sulfilimine [R1 R2 (S=NH)] and/or sulfoximine [R1 R2 S(O)(=NH)] derivatives. In the asymmetric synthesis and chemical modification of p-bromophenyl methyl sulfoximine, O-mesitylenesulfonylhydroxylamine (MSH) could realize stereospecific imination[3].
References
[1] Guo Z, et al. Precise identification of O-linked β-N-acetylglucosamine peptides based on O-mesitylenesulfonylhydroxylamine elimination reaction. Chinese Journal of Chromatography, 2021; 39: 1182 - 1190.
[2] Kitowski A, et al. Oxidative Activation of C–S Bonds with an Electropositive Nitrogen Promoter Enables Orthogonal Glycosylation of Alkyl over Phenyl Thioglycosides. Organic Letters, 2017; 19: 5490–5493.
[3] Cho G, et al. Correction to Synthesis and Palladium-Catalyzed Coupling Reactions of Enantiopurep-Bromophenyl Methyl Sulfoximine. The Journal of Organic Chemistry, 2010; 75: 522.
);