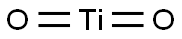Titanium Dioxide: A Review on Applications, Impact, and Toxicity
General Description
Titanium dioxide, also known as titanium(IV) oxide or titania also known as Titania belongs to the family of transition metal oxides, is the naturally occurring oxide of titanium, chemical formula TiO2. Titanium dioxide was first manufactured a century ago and is significant in industry due to its chemical inertness, low cost, and availability. The white mineral has a wide range of applications in photocatalysis, in the pharmaceutical industry, and in food processing sectors. Titanium dioxide is a white color found in all kinds of paints, printing ink, plastics, paper, synthetic fibers, rubber, condensers, painting colors and crayons, ceramics, electronic components along with food and cosmetics. When used as a pigment, it is called titanium white, Pigment White 6, or CI 77891. Titanium dioxide exists naturally in three crystalline forms; anatase, rutile and brookite. The great efforts devoted to the research on Titanium dioxide material produced many promising uses in areas which range from photovoltaics and photocatalysis to photo-electrochromics and sensors.1-3

Figure 1. Properties of Titanium dioxide
Applications
Titanium dioxide's uses can be generally classified into "energy" and "environmental" types, many of types rely not only on the properties of the Titanium dioxide material itself but also on the changes in the Titanium dioxide material host.
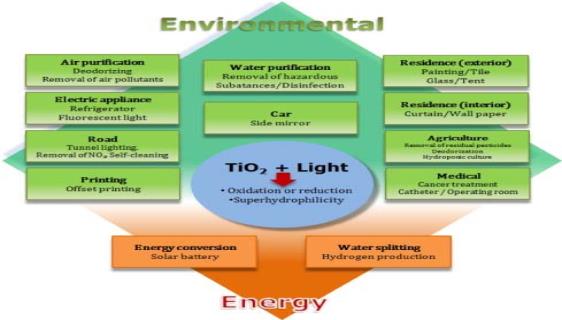
Figure 2. Applications of Titanium Dioxide
Titanium Dioxide as Photocatalysis
Photo-catalysis is the composing of photochemistry and catalysis with both light and a catalyst being desired to onset or precipitate a chemical conversion. The photo-catalytic process starts with the absorption of electromagnetic radiation, which excites an electron from the valence band to the conduction band, leaving a hole in the valence band. In this process UV light irradiation is used by photon energy equal to or greater than Titanium dioxide band gap energy (hν≥ 3.20 eV at λ ≤ 380 nm); electron-hole pairs (The charge carrier) are generated. The negatively charged electron moves from the valance band (VB) to the conduction band (CB) leaving behind the positively charged hole. Then, the electron and hole take part in reduction oxidation reactions with species that are adsorbed on the surface of Titanium dioxide, such as water, hydroxide (OH-) ions, organic compounds or oxygen. The valence band hole (h+) is highly oxidizing while the conduction band electron (e-) is highly reducing. The charge carrier h+ oxidizes H2O or OH- ion to the hydroxyl radical (OH•) that is a highly potent, non-selective oxidant. It easily attacks pollutants adsorbed at the surface of titanium dioxide or in aqueous solution degrading them to H2O and CO2. On the conduction band (CB) the electron reduces adsorbed oxygen (O2) species to superoxide (O2•), then undergoes a series of reactions to give the OH• radical. The reaction of these radicals with organic substance, environmental pollutants or harmful microorganisms results in the decomposition of the latter.4
Antibacterial Activity of Titanium Dioxide
Photocatalytic titanium dioxide antimicrobial effect reaction was first discovered by. They investigated the effectiveness of the photocatalytic oxidation under UV irradiation against numerous microorganisms of Gram-positive bacteria (Lactobacillus acidophilus), yeast (Saccharomyces cerevisiae), Gram-negative bacteria (Escherichia coli) and green algae (Chlorella vulgaris). Since then, a series of investigations on photocatalytic disinfection have been intensively conducted on a wide range of microorganisms such as viruses, fungi and many species of bacteria.UV light irradiation of titanium dioxide activates valence band electrons to be transferred to the conduction band leaving behind a positively charged hole. The activated charge carriers react with atmospheric oxygen and water molecules to produce reactive oxygen species (ROS). Biocidal action of titanium dioxide photocatalyst is frequently ascribed to OH• radicals and other reactive oxygen species (ROS) which is the driving force behind the antibacterial activity of titanium dioxide.In particular, some studies have proved that the cell membrane is the fundamental site to be attacked by reactive photogenerated oxygen species, leading to lipid peroxidation. The combination of cell membrane damage and more oxidative attack of intracellular components eventually caused cell death. Other studies have propose that the photo oxidation of coenzyme A (a coenzyme derived from pantothenic acid, important in respiration and many other biochemical reactions) will leading to cell respiration inhibition and finally cell death. Generally, disinfections by titanium oxide are 3 times stronger than chlorine and 1.5 times stronger than ozone.5
Titanium Dioxide as A Food Addictive
Consumer trends and dietary habits are diverse and changing at a fast pace. These are mainly influenced by modern lifestyle choices and by societal demands for a diversity of new flavours. Consequently, safe preservatives are necessary for tackling the waste of fresh foods and beverages, while enhancing their physical properties. In the food processing sector, titanium dioxide comes under the name E171 in the European Union and INS171 in the United States. The name "titanium white" is also significant, because the metal oxide powder increases the opacity, brightness, and whiteness of many foods. As such, it is authorised for use in creamer formulations, to thicken their texture, and in fish, instant drinks, and confectionary products, to enhance their flavour. It was also found to improve the taste of many coloured foods incorporated into a daily diet. Despite its presence in many foods, E171 is an additive with no nutritional value.6
Titanium Dioxide Toxicity
Late-20th century publications on titanium dioxide's toxicity exposed a correlation between inhaling fine particles of this mineral and the emergence of pleural diseases, especially in workers exposed to it in manufacturing plants. At present, titanium dioxide is classified by the International Agency for Research on Cancer (IARC) as a 2B type carcinogen, equivalent to "possibly carcinogenic to humans". This classification also implies that further studies are required to fully ascertain its cytotoxic and genotoxic potential.7
For almost a decade, oral ingestion of nano titanium dioxide has represented a concerning health issue for the population. Studies on rodents and human volunteers indicate that once titania passes the intestinal barrier, it reaches the bloodstream and is then transported to vital organs. If oral consumption of the titanium dioxide's nanoform exceeds the recommended limit, it can accumulate in tissues and induce irreversible damage. The harmful effects observed on rodents include stomach and intestine inflammation, liver cell necrosis, lesions to cardiovascular tissue, enhancement of anxiety disorder, progression of colon tumours, and nephrotoxicity in kidneys. There are many experimental variables that can influence the outcome of ingestion toxicity assays, but the main ones to consider are the concentration and reactivity of titanium dioxide nanoparticles.8
References
1. Braun, J.H.; Baidins, A.; Marganski, R.E. TiO2 pigment technology: A review. Prog. Org. Coat. 1992, 20, 105–138.
2. Shand, M.; Anderson, A.J. Aqueous phase photocatalytic nitrate destruction using titania based materials: Routes to enhanced performance and prospects for visible light activation. Catal. Sci. Technol. 2013, 3, 879–899.
3. Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906.
4. Linsebigler, A. L., Lu, G., & Yates, J. T. "Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results" Chemical Reviews, 95(3) (1995): 735–758.
5. Allahverdiyev, A. M., Abamor, E. S., Bagirova, M., & Rafailovich, M. "Antimicrobial effects of TiO(2) and Ag(2)O nanoparticles against drugresistant bacteria and leishmania parasites" Future Microbiology, 6(8) (2011): 933–940.
6. Zink, D.L. The impact of consumer demands and trends on food processing. Emerg. Infect. Dis. 1997, 3, 467–469.
7. ARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 93, pp. 1–413.
8. Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; Díaz-Urbina, D.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; González, M.I.; Reyes, J.L.; Villamar-Duque, T.E.; Flores-Sánchez, M.L.; Hernández-Pando, R.; et al. Food-grade titanium dioxide (E171) induces anxiety, adenomas in colon and goblet cells hyperplasia in a regular diet model and microvesicular steatosis in a high fat diet model. Food Chem. Toxicol. 2020, 146, 111786.
);You may like
Related articles And Qustion
See also
Lastest Price from Titanium dioxide manufacturers

US $1900.00-1100.00/T2024-04-26
- CAS:
- 13463-67-7
- Min. Order:
- 0.10000000149011612T
- Purity:
- 98%
- Supply Ability:
- 20T

US $1850.00-1200.00/T2024-04-26
- CAS:
- 13463-67-7
- Min. Order:
- 0.10000000149011612T
- Purity:
- 98%
- Supply Ability:
- 100T