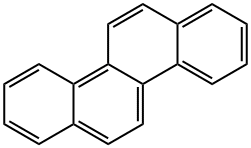
Toxicity of Chrysene
Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C18H12. It is one of the natural constituents in coal tar, from which it was first isolated and characterized. It is produced as a gas during combustion of coal, gasoline, garbage, animal, and plant materials and usually found in smoke and soot. Chrysene usually combines with dust particles in the air and is carried into water and soil and onto crops. Creosote, a chemical used to preserve wood contains chrysene. High concentration of chrysene in the air is typically found during open burning and home heating with wood and coal. People are exposed to chrysene from a variety of environmental sources such as air, water, and soil and from cigarette smoke and cooked food. General population is usually exposed to chrysene along with a mixture of similar chemicals.

Property
Chrysene is a by-product of many industrial processes and thereby released in the atmosphere. Chrysene is lipophilic, insoluble in water, slightly soluble in other polar solvents such as alcohol, ether and moderately soluble in benzene and toluene. However, it readily dissolves in benzene and toluene at an elevated temperature. The name ‘Chrysene’ originates from the Greek word chrysos, meaning ‘gold,’ and is due to the golden yellow color of the slightly impure crystals. However, in pure state, chrysene is a colorless, crystalline solid. It has characteristic red–blue fluorescence under UV light. Some important properties of chrysene are summarized below.
Uses
Chrysene is used strictly for research purposes.
Environmental fate
Generally, disposal of PAH from the industrial plants, accidental release from the containers, smoke from plant, combustion, or automobile exhaust causes chrysene and other PAHs to enter the environment. Because of the poor water solubility and low vapor pressure, chrysene has limited chance to get washed away or evaporate in the environment. Therefore, it remains immobile in soils. If exposed to water, it gets absorbed on the particulate matters and either float or sediment on the riverbed. The rate of biodegradation in soil ranges from 77 to 387 days depending on the soil type.
Chrysene does not undergo hydrolysis due to the lack of hydrolyzable functional groups. However, it undergoes photochemical oxidations when exposed to the environment. Dihydrodiol is the common degradation product of chrysene. Half-life of degradation of chrysene, absorbed to soot particles and exposed to sunlight in air containing 10 ppm nitrogen oxides is 26 days. The National Research Council (NRC 1983) noted that the PAHs adsorbed to soot particles are more resistant to photochemical reactions than pure compounds.
Toxicity
Compounds under the class of PAH are potent carcinogens. Carcinogenicity of chrysene is due to the mutagenic activity of its metabolites, 1,2-dihydrodiol and 1,2-diol-3,4-epoxide. Compounds containing the bay-region (sterically hindered cupshaped area between carbon 10 and 11 of benzo[a]pyrene or 1 and 12 of benzo[a]anthracene) in their structure are considered to be the potent carcinogen because of their reactivity to enzymes. Chrysene has a bay-region in its structure, which is metabolized by mixed function oxidases to reactive ‘bay-region’ diol epoxides. These diol epoxides get converted to carbocations, which are mutagens and initiators of carcinogenesis. This compound together with other PAHs may be more toxic or carcinogenic than a single compound.
It has been reported that the heavier PAHs (4,5 and 6-rings) such as chrysene tend to be greater carcinogenic than lighter PAHs. Within the aromatic series, acute toxicity increases with increasing alkyl substitution on the aromatic nucleus. The metabolites of chrysene, 1,2-dihydrodiol and 1,2-diol-3,4-epoxide are mutagenic in in vitro mammalian and bacterial cell cultures and have induced pulmonary adenomas in newborn mice.
In addition, the 1,2-dihydrodiol was a tumor-initiating agent in mouse skin. All PAHs act as carcinogens through biotransformation to chemically relevant intermediates that covalently bind to cellular macromolecules such as DNA, which leads to mutation and tumor initiation. They also induce P450 isoenzyme, important for the metabolism of PAHs.
);You may like
Lastest Price from Chrysene manufacturers

US $15.00-10.00/KG2021-07-13
- CAS:
- 218-01-9
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton

US $15.00-10.00/KG2021-07-09
- CAS:
- 218-01-9
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton