triacetate is a common plasticizer
General description
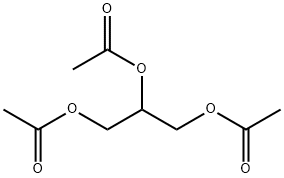
Triacetin is a triglyceride obtained by acetylation of the three hydroxy groups of glycerol. It has fungistatic properties (based on release of acetic acid) and has been used in the topical treatment of minor dermatophyte infections. It has a role as a plant metabolite, a solvent, a fuel additive, an adjuvant, a food additive carrier, a food emulsifier, a food humectant and an antifungal drug. It derives from an acetic acid. Triacetin is a natural product found in Vitis vinifera with data available. LOTUS - the natural products occurrence database A triglyceride that is used as an antifungal agent[1].
Figure 1 the chemical structure of Triacetin
Application and Pharmacology
Glyceryl triacetate is a common plasticizer for acetate fiber filter rod. Its dosage directly affects the hardness, pressure drop and head shrinkage of the filter rod [1-2], and then affects the filtration efficiency of the filter rod and the suction quality of cigarettes. Therefore, the tobacco industry promulgated and implemented the standard of glyceryl triacetate for tobacco (yc144-2008) [3] in 2008, which requires the purity of glyceryl triacetate for tobacco to reach more than 99%.
1.Triacetin, the water-soluble triglyceride of acetate, was infused in mongrel dogs at isocaloric (N=6) or hyper- caloric (~1.5 REE, N =7) rates in mongrel dogs for 3 hr. Ketone body and glucose production rates were quantified with acetoacetate and glucose, respectively. Four additional animals were infused with glycerol to serve as controls for the hypercaloric triacetin infusion. Energy expenditure was determined in the isocaloric experiments. Results: no evidence of acute toxicity was observed during triacetin infusion at either rate. Plasma acetate concentrations increased from basal levels to ~1 and ~13 mmol/liter in the isocaloric and hypercaloric experiments, respectively. Plasma lactate and pyruvate concentrations decreased dramatically after 30 min of both isocaloric and hypercaloric triacetin infusions. Glucose production rates did not increase in either group, but glucose clearance decreased significantly in both groups (p<0.05) over the last hour of triacetin infusion. Plasma ketone body concentrations in- creased from 1.4 to 3.5 and 1.8 to 13.5 min, respectively, during isocaloric and hypercaloric triacetin infusion. The decrease in glucose clearance may represent competition between carbohydrate (glucose) and lipid (acetate). Triacetin infusion resulted in significant increases in ketone body production and concentration. These preliminary data indicate that triacetin may have a future role as a parenteral nutrient, and that further studies of its use are warranted[2].
2.Plasticization effect of triacetin on structure and properties of starch ester film:The plasticizing effect of triacetin on the structure and properties of starch ester film and further establish the structure–property relationships. The presence of triacetin resulted in multiple structure changes of the film. The mobility of macromolecular chain was increased to form scattered crystallite during the film formation process. The amorphous region was enlarged to contain more triacetin squeezed from crystalline region. The plasticization of triacetin and restriction of crystallite oppositely influenced the mobility of macromolecular chains in different regions. The thermal stability of triacetin changed along with its fluctuant interaction with macromolecules. Comparatively, the enhanced ether bond and the restriction from crystalline regions on the mobility of the amorphous chain consequently improved the thermal stability of the film matrix. The interaction between triacetin and starch ester was essential to film forming but unexpectedly lowered the triacetin stability[3].
Synthesis
The waste liquid of biodiesel enzymatic production process (containing glycerol 5%) After alkali treatment - solvent removal by vacuum distillation - glue removal - vacuum distillation Glycerol with purity of 90.43% was obtained. The extracted glycerol reacted with glacial acetic acid The reaction conditions are as follows: phosphotungstic acid as catalyst Toluene as water carrying agent Temperature 110 ℃ The material ratio of glycerol to glacial acetic acid is 1 ∶ 5 The amount of catalyst used is 1.6% of the raw glycerol The reaction time is 6h. The yield of glycerol triacetate can reach more than 90%[4].
Safety
Triacetin, also known as Glyceryl Triacetate, is reported to function as a cosmetic biocide, plasticizer, and solvent in cosmetic formulations, at concentrations ranging from 0.8% to 4.0%. It is a commonly used carrier for flavors and fragrances. Triacetin was affirmed as a generally recognized as safe (GRAS) human food ingredient by the Food and Drug Administration (FDA). Triacetin was not toxic to animals in acute oral or dermal exposures, nor was it toxic in short-term inhalation or parenteral studies, and subchronic feeding and inhalation studies. Triacetin was, at most, slightly irritating to guinea pig skin. However, in one study, it caused erythema, slight edema, alopecia, and desquamation, and did cause some irritation in rabbit eyes. Triacetin was not sensitizing in guinea pigs. Triacetin was not an irritant or a sensitizer in a clinical maximization study, and only very mild reactions were seen in a Duhring-chamber test using a 50% dilution. In humans, Triacetin reportedly has caused ocular irritation but no injury. Triacetin was not mutagenic. Although there were no available reproductive and developmental toxicity data, Triacetin was quickly metabolized to glycerol and acetic acid and these chemicals were not developmental toxins. Reports of 1,2-glyceryl diesters, which may be present in Triacetin, affecting cell growth and proliferation raised the possibility of hyperplasia and/or tumor promotion. The Cosmetic Ingredient Review (CIR) Expert Panel concluded, however, that the effects of 1,2-glyceryl diesters on cell growth and proliferation require longer ester chains on the glycerin backbone than are present when acetic acid is esterified with glycerin, as in Triacetin. On the basis of the available information, the CIR Expert Panel concluded that Triacetin is safe as used in cosmetic formulations[5].
References
1.Kong Haohui, Chen Cuiling, Wang Junxia, etc.: Research on the detection method of glycerol triacetate, modern food science and technology, 2010, No. 02, pp. 215-217.
2.Zhu J., Li X. & Huang C. et al., "Plasticization effect of triacetin on structure and properties of starch ester film," Carbohydrate Polymers, Vol.94, No.2(2013), pp.874-881.
3.Tsen A. R., Long P. M. & Driscoll H. E. et al., "Triacetin-based acetate supplementation as a chemotherapeutic adjuvant therapy in glioma," International Journal of Cancer, Vol.134, No.6(2014), pp.1300-1310.
4.Guo Xiaoya, CI Bingbing, Yu Jinglu, etc.: extraction of glycerol from biodiesel production waste liquid and synthesis of glycerol triacetate, Journal of Shanghai University (NATURAL SCIENCE EDITION), 2010, No. 05, pp. 522-525.5.Bailey J. W., Haymond M. W. & Miles J. M., "Triacetin: A Potential Parenteral Nutrient," JPEN. Journal of parenteral and enteral nutrition, Vol.15, No.1(1991), pp.32-36.
);You may like
Lastest Price from Triacetin manufacturers

US $10.00/kg2024-04-25
- CAS:
- 102-76-1
- Min. Order:
- 1kg
- Purity:
- 99.8%
- Supply Ability:
- 10000ton

US $3.00/kg2024-04-20
- CAS:
- 102-76-1
- Min. Order:
- 1kg
- Purity:
- 99.92%
- Supply Ability:
- 50000tons