Uses and Adverse effect of Gabapentin
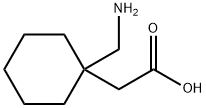
Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) that was first approved for use in the United States in 1993 (1) It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures (2) - today it is also widely used to treat neuropathic pain. Gabapentin has some stark advantages as compared with other anti-epileptics, such as a relatively benign adverse effect profile, wide therapeutic index, and lack of appreciable metabolism making it unlikely to participate in pharmacokinetic drug interactions (1, 2). It is structurally and functionally related to another GABA derivative, pregabalin.
Uses
In the United States, gabapentin is officially indicated for the treatment of postherpetic neuralgia in adults and for the adjunctive treatment of partial-onset seizures, with or without secondary generalization, in patients 3 years of age and older (1,2). In Europe, gabapentin is indicated for adjunctive therapy in the treatment of partial-onset seizures, with or without secondary generalization, in patients 6 years of age and older and as monotherapy in patients 12 years of age and older. It is also used in adults for the treatment of various types of peripheral neuropathic pain, such as painful diabetic neuropathy (3).
Pharmacodynamic
Gabapentin is an anti-convulsant medication that inhibits the release of excitatory neurotransmitters, allowing for its use against pathologic neurotransmission such as that seen in neuropathic pain and seizure disorders (1-3). It has a wide therapeutic index, with doses in excess of 8000 mg/kg failing to cause a fatal reaction in rats (1-3).
Gabapentin is ineffective in absence seizures and should be used in caution in patients with mixed seizure disorders involving absence seizures. Gabapentin has been associated with drug reaction with eosinophilia and systemic symptoms (DRESS), otherwise known as multi-organ hypersensitivity. This reaction can prove fatal and early symptoms such as fever, lymphadenopathy, and rash should be promptly investigated (1-3)
Mechanism of action
The precise mechanism through which gabapentin exerts its therapeutic effects is unclear (1-3). The primary mode of action appears to be at the auxillary α2δ-1 subunit of voltage-gated calcium channels (though a low affinity for the α2δ-2 subunit has also been reported) (4-6). The major function of these subunits is to facilitate the movement of pore-forming α1 subunits of calcium channels from the endoplasmic reticulum to the cell membrane of pre-synaptic neurons (5). There is evidence that chronic pain states can cause an increase in the expression of α2δ subunits and that these changes correlate with hyperalgesia (4). Gabapentin appears to inhibit the action of α2δ-1 subunits, thus decreasing the density of pre-synaptic voltage-gated calcium channels and subsequent release of excitatory neurotransmitters (5). It is likely that this inhibition is also responsible for the anti-epileptic action of gabapentin (6).
There is some evidence that gabapentin also acts on adenosine receptors (7) and voltage-gated potassium channels (8), though the clinical relevance of its action at these sites is unclear.
Absorption, volume of distribution, metabolism and elimination
Absorption of gabapentin is thought to occur solely via facilitated transport by the LAT1 transporter within the intestines.5 As this process is saturable, the oral bioavailability of gabapentin is inversely proportional to the administered dose - the oral bioavailability of a 900mg/day regimen is approximately 60%, whereas a 4800mg/day regimen results in only 27% bioavailability (1-3). The Tmax of gabapentin has been estimated to be 2-3 hours (4-6). Food has no appreciable effect on gabapentin absorption (1-3).
The apparent volume of distribution of gabapentin after IV administration is 58±6 L. The drug is found in the CSF in concentrations approximately 9-20% of the corresponding plasma concentrations and is secreted into breast milk in concentrations similar to that seen in plasma (2-4).
Gabapentin is not appreciably metabolized in humans (2,3) - in humans, metabolites account for less than 1% of an administered dose, with the remainder being excreted as unchanged parent drug in the urine.5
Gabapentin is eliminated solely in the urine as unchanged drug (2,3). Cimetidine, an inhibitor of renal tubular secretion, reduces clearance by approximately 12%, suggesting that some degree of tubular secretion is involved in the renal elimination of gabapentin (2).
The elimination t1/2 of gabapentin in patients with normal renal function is 5-7 hours (2-4). In patients with reduced renal function, the elimination t1/2 may be prolonged - in patients with a creatinine clearance of <30 mL/min, the reported half-life of gabapentin was approximately 52 hours (1,2).
Adverse effect and tolerance
The oral TDLo of gabapentin in humans is 2.86 mg/kg and the LD50 in rats has been found to be >8000 mg/kg (3). Symptoms of overdose are consistent with the drug's adverse effect profile and involve CNS depression (e.g. dizziness, drowsiness, slurred speech, lethargy, loss of consciousness) and gastrointestinal symptoms such as diarrhea (1-3). Management of overdose should involve symptomatic and supportive treatment. Gabapentin can be removed by hemodialysis - this may be of benefit in some patients, such as those with impaired renal function (2).
Multi-drug overdoses involving gabapentin, particularly in combination with other CNS depressants such as opioids, can result in coma and death - this possibility should be considered when managing overdosage (3).
Gabapentin is tolerated safely over a very broad range of doses from approximately 800 to 1800 mg/day (although package inserts suggest that patients may be treated with doses as high as 3600 mg/day). In clinical practice, dosing is typically titrated starting from lower doses (i.e. < 400 mg/day) and moving rapidly upward. The EMA [9] and the Physician Prescribing Information generally recommends dosing up to 1800 mg in adults. While substantially higher doses have been tested in clinical trials, no additional clinical benefit has been observed [10].
However, other studies have examined gabapentin as acute doses in the higher dose range, and it was well tolerated. At least one imaging study has reported that gabapentin (1200 and 2400 mg) significantly (and rapidly) increased measurable concentrations of brain GABA, one of its presumed mechanisms of action. Hart and colleagues [11] examined gabapentin (600 and 1200 mg) for its potential to reduce the reinforcing effects of cocaine in the human laboratory. Their data reveal reductions in ratings of anxiety with both gabapentin doses (in the absence of cocaine) compared to placebo. Lile [12] examined 600 and 1200 mg yielding significant differences from placebo on numerous outcomes, including liking, take again and good effects. Bisaga & Evans [13] examined gabapentin in combination with alcohol at acute doses of 1000 and 2000 mg. In this dose range, gabapentin produced some direct effect on psychomotor function but was still tolerated safely in combination with alcohol.
References
1. Bockbrader HN, et al Clin Pharmacokinet. 2010 Oct;49(10):661-9.
2. FDA Approved Drug Products: Neurontin (gabapentin) for oral use
3. EMA Approved Drugs: Gabapentin
4. Maneuf YP, Luo ZD, Lee K: Semin Cell Dev Biol. 2006 Oct;17(5):565-70.
5. Kukkar A, Bali A, Singh N, Jaggi AS: Arch Pharm Res. 2013 Mar;36(3):237-51.
6. Sills GJ: Curr Opin Pharmacol. 2006 Feb;6(1):108-13.
7. Martins DF, et al J Peripher Nerv Syst. 2015 Dec;20(4):403-9.
8. Manville RW, Abbott GW: Mol Pharmacol. 2018 Oct;94(4):1155-1163.
9. European Medicines Agency (EMA). Annex III: Summary of Product Characteristics, Labelling, and Package Leaflet. London, UK: EMA; 2006.
10. Pfizer. Neurontin U.S. Physician Prescribing Information. New York, NY: Pfizer; 2014.
11. Hart C. L., et al Drug Alcohol Depend 2004; 73: 279–87.
12. Lile J. A., Kelly T., Hays L. Separate and Combined Effects of Gabapentin and Δ 9-THC Doses in Cannabis Users Discriminating Δ 9-THC. San Diego, CA: College on Problems of Drug Dependence; 2013.
13. Bisaga A., Evans S. M. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83: 25–32.
);You may like
Related articles And Qustion
See also
Lastest Price from Gabapentin manufacturers

US $1.00/ASSAYS2024-05-04
- CAS:
- 60142-96-3
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1kg

US $50.00-30.00/kg2024-04-30
- CAS:
- 60142-96-3
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 1000