Uses of Chlorothalonil
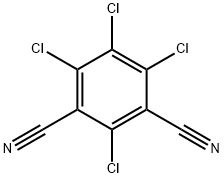
Chlorothalonil is an aromatic halogen compound that appears as a grayish to colorless crystalline solid that is odorless or has a slightly pungent odor. It has a molecular weight of 265.92, water solubility of 0.6 mg l-1 at room temperature, a melting point of 250–251°C, and a boiling point of 350°C. Chlorothalonil is only slightly soluble in acetone, dimethyl sulfoxide, cyclohexane, and xylene. It is noncorrosive and stable in moderately alkaline or acidic aqueous solutions. At high temperatures, chlorothalonil decomposes to emit hydrochloric acid. Some popular trade names for chlorothalonil include Bravo, Daconil 2787, Echo, Exotherm Termil, Nopcocide, Repulse, and Tuffcide. This compound can be found in formulations with many other pesticide compounds.

Uses
Chlorothalonil is an important broad-spectrum, nonsystemic, organochlorine fungicide that has been widely used for more than 30 years as an effective disease management tool for potatoes, peanuts, turf, and vegetable and fruit crops. It is also used to control fruit rots in cranberry bogs and is used in paints. Chlorothalonil is classified as a general use pesticide by the US Environmental Protection Agency (EPA). Typical chlorothalonil application rates are 1 kg ha-1 with four to nine applications per growing season. Chlorothalonil can enter surface waters through rainfall runoff, spray drift, or atmospheric deposition, subsequently having an impact on the aquatic biota. Most common routes of exposure to chlorothalonil are via the skin and eyes; it may also be ingested or inhaled.
Environmental Fate
Chlorothalonil’s production and use as a broad-spectrum, nonsystemic, protectant pesticide results in its direct release to the environment. Its uses as a wood protectant, antimold and antimildew agent, bactericide, microbiocide, algaecide, insecticide, and acaricide are additional routes of release. If released to air, chlorothalonil will exist in both the vapor and particulate phases in the ambient atmosphere. Vapor-phase chlorothalonil will be degraded slowly in the atmosphere by reaction with photochemically produced hydroxyl radicals (reaction half-life ~7 years). Direct photolysis may also occur. Chlorothalonil is removed from the atmosphere by wet and dry deposition. If released to soil, chlorothalonil is expected to have lowmobility or be immobile, based on Koc values in the range of 900–7000 measured in four soils. Volatilization from moist or dry soil surfaces is not expected to be important based on a Henry’s Law constant of 2.5×10-7 atm-cummol-1. Aerobic biodegradation half-lives of chlorothalonil in four different soils ranged from 10 to 40 days. If released into water, chlorothalonil is expected to adsorb to suspended solids and sediment in the water column.
Mechanism of Toxicity
Glutathione conjugation represents a bioactivation reaction for chlorothalonil resulting in the formation of S-conjugates toxic to the kidney. Chlorothalonil acts as an alkylating agent and reacts with cellular sulfhydryl compounds. Alkylation of biological molecules results in effects on cellular function and viability. Chronic damage to the proximal tubular epithelium may be involved in the mechanism of chlorothalonil tumorigenicity to the kidneys.
);You may like
Lastest Price from Chlorothalonil manufacturers

US $0.00-0.00/kg2024-04-03
- CAS:
- 1897-45-6
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 20 tons

US $2.00/Kg/Drum2023-12-12
- CAS:
- 1897-45-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20KL per month