What is 17-AAG?
Description
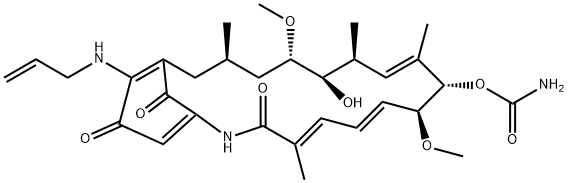
Tanespimycin, also known as 17-AAG, CP127374, NSC-330507, or 17-allylamino-17-demethoxygeldanamycin, is an Hsp90 inhibitor[1]. This drug is an organic compound with the chemical formula C31H43N3O8. It is a purple or dark red solid. This compound is a synthetic analog of geldanamycin, an antibiotic first purified in 1970 from Streptomyces hygroscopicus.

History
In the early 1990s, in vitro and in vivo experiments demonstrated that geldanamycin has antitumor activity against various human-derived tumor cell lines. In addition, further in vitro experiments showed that geldanamycin binds the 90 kDa heat shock protein (HSP90), inhibiting the formation of a src-HSP90 complex. The drug was later found to cause significant hepatic toxicity in animal studies. Hence, Geldanamycin analogs such as 17-AAG were developed.
Mechanism of action
17-AAG binds to HSP90 and destabilizes client proteins in tumor cells (ie, decreases in HER2 and Raf-1, instability in p53, and interruptions in MAPK signaling). Tumor-derived HSP90 has a 100-fold higher affinity for tanespimycin compared with HSP90 derived from normal cells, suggesting that tumor cells contain an activated HSP90 conformation that might help tumorigenesis and appear to be a susceptible anticancer target. HSP90 inhibitors kill multiple myeloma (MM) cells partly by inducing endoplasmic reticulum stress (leading to JNK activation and caspase-dependent apoptosis) and inducing the unfolded protein response. This drug is cytotoxic to primary MM cells and MM cell lines, including cell lines resistant to conventional chemotherapy. Tanespimycin can synergize with the proteasome inhibitor bortezomib to induce apoptosis in primary MM cells resistant to doxorubicin and bortezomib. Given in combination with bortezomib, tanespimycin has a synergistic apoptotic effect on MM cell lines[2].
Safety
17-AAG in combination with bortezomib (using both the Cremophor-based formulation and suspension formulation) in 72 patients with relapsed and refractory MM. The most frequent adverse events were diarrhea (60%), nausea (49%), fatigue (49%), thrombocytopenia (40%), and reversible elevation of aspartate aminotransferase (AST; 28%). The most common grade 3/4 toxicities included thrombocytopenia (25%) and diarrhea, anemia, and fatigue (7% each, respectively)[2]. The finding that none of the patients experienced grade 3/4 peripheral neuropathy appears to be consistent with the neuroprotective effect seen with tanespimycin in some preclinical cancer models.
References
[1] Gupta B, et al. Folate receptor-targeted hybrid lipid-core nanocapsules for sequential delivery of doxorubicin and tanespimycin. Colloids and Surfaces B: Biointerfaces, 2017; 155: 83-92.
[2] Dimopoulos M, et al. Tanespimycin as antitumor therapy. Clinical Lymphoma Myeloma & Leukemia, 2011; 11: 17-22.
);