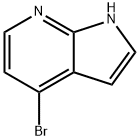
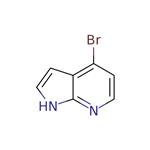
What is 4-Bromo-7-azaindole?
Feb 18,2020
4-Bromo-7-azaindole is an important organic intermediate (building block) to synthetize substituted azaindole products.
The following example is about its application on the synthesis of 4-Bromo-1-(toluene-4-sulfonyl)-1H-pyrrolo[2,3-b]pyridine [1].
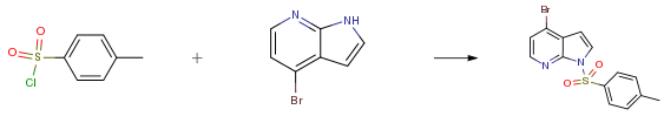
4-Bromo-7-azaindole (3 g, 15.22 mmol) was weighed into a round bottom flask and dissolved in THF (50 mL) under nitrogen. The reaction mixture was cooled to 0° C. and treated portionwise with sodium hydride (60% in mineral oil, 0.67 g, 16.75 mmol), the addition was accompanied by fizzing. After the addition the reaction mixture was allowed to stir for 30 minutes at room temperature and then treated with benzenesulfonyl chloride (2.14 mL, 16.75 mmol). The reaction mixture was allowed to warm to room temperature and stirred for 2 hours. The reaction mixture was evaporated under reduced pressure and dissolved in DCM 30 mL, the organics were washed with 2×30 mL portions of 2M sodium carbonate, dried with MgSO4, filtered and evaporated to an orange oil. Purified by flash column chromatography eluting with 1:9 ethyl acetate:cyclohexane to provide the final compound as an off white solid (92%).
The following example is about its application on the synthesis of 4-bromo-1-[tris(propan-2-yl)silyl]-1 H-pyrrolo[2,3-b]pyridine [2].
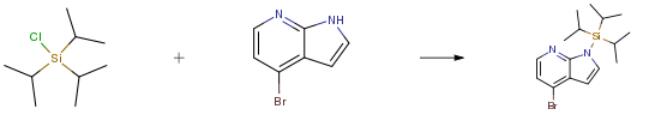
Sodium hydride, 60% dispersion in mineral oil (0.49g, 12.l8mmol) was added in portions to 4-bromo-IH-pyrrolopyridine (2g, 10.l mmol) in THF (45mL) at 0°C and stirred for 15 minutes. Triisopropylsilyl chloride (2.31g, 2.57mL, l2mmol) was then added to the reaction mixture drop wise at 0°C. After addition the cooling was removed and the reaction mixture allowed to attain RT, where it was stirred for a further 1 hour. The suspension was then cooled to about 0-5°C and quenched with saturated aqueous ammonium chloride (30 mL). The aqueous phase was extracted with EtOAc (3×30 mL) and the combined organic phases were washed with saturated aqueous sodium chloride, dried (MgSO4) and solvent removed in vacuo to afford a yellow oil. The crude material was purified by flash chromatography, eluting with iso-hexane. Fractions found to contain pure product were combined and solvent removed in vacuo to afford the desired compound as an oil, 3.5g, 97.6%.
The following example is about its application on the synthesis of 4-[1-ethyl-3-(4-nitrophenyl)-1H-pyrazol-4-yl]-1H-pyrrolo[2,3-b]pyridine [3].
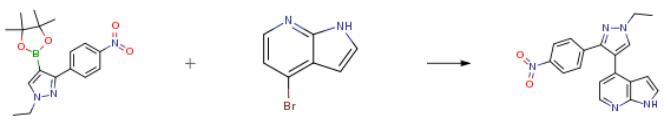
1-ethyl-3-(4-nitrophenyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol(7.5 mmol), 4-bromo-1H-pyrrolo[2,3-b]pyridine (6.3 mmol), and tetrakis(triphenylphosphine)palladium(0) (0.25 mmol) in a 1:1 solution of 1,4-dioxane (12 mL):2M potassium carbonate (12 mL) was stirred for 18 h at 100° C. in a sealed tube. Upon cooling to room temperature, product precipitated out of solution which was filtered and dried to provide the final product as a light yellow powder (80 percent).
The following example is about its application on the synthesis of pyrazinyl carboxamide derivatives [4].
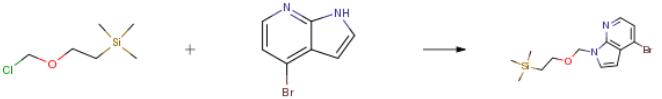
4-bromo-l-{[2-(trimethylsilyl)ethoxy]methyl}-lH-pyrrolo[2,3-b]pyridine4-Bromo-lH-pyrrolo[2,3-b]pyridine (10.0 g, 0.0508 mol,) was dissolved in N,N-dimethylformamide (100 mL) and cooled under nitrogen to 0 °C. Sodium hydride (3.00 g, 0.0750 mol, 60 percent disperson in mineral oil) was added portion-wise. The reaction was stirred for 10 minutes, [β- (Trimethylsilyl)ethoxy]methyl chloride (10.8 mL, 0.0609 mol) was added slowly to the reaction mixture, stirred at 0 °C for 45 minutes, and allowed to warm to room temperature. The solvent was removed under reduced pressure. The residue was diluted with ethyl ether (100 mL), and washed with water and brine, dried over sodium sulfate and concentrated under reduced pressure. The residue was purified by flash chromatography on a silica gel column with ethyl acetate in hexane (0 - 25percent) to afford the desired product (16.04 g, 96.6percent).
References
1.Ignyta, Inc. Cancer Research Technology Limited; Dorsey BD, Dugan BJ, Fowler KM, Hudkins RL, Mesaros EF, Zificsak CA, Zulli AL. Azaquinazoline inhibitors of atypical protein kinase C. US2015/274720[P], 2015, A1, Location in patent: Paragraph 0202.
2.Vernalis (R&D) Limited. Stokes S, Graham CJ, Ray SC, Stefaniak EJ. 1H-pyrrolo[2,3-b] pyridine derivatives and their use as kinase inhibitors. WO2013/114113[P], 2013, A1, Page/Page column 29; 30.
3.Dhanak D, Newlander KA. Azaindole inhibitors of aurora kinases. S2007/149561[P], 2007, A1, Page/Page column 85.
4.Incyte Corporation. Yao W, Burns DM, Zhuo J. Azetidinyl phenyl, pyridyl or pyrazinyl carboxamide derivatives as JAK inhibitors. WO2012/177606[P], 2012, A1, Page/Page column 54.
);
Related articles And Qustion
See also
Lastest Price from 4-Bromo-7-azaindole manufacturers
4-Bromo-7-azaindole
US $1750.00/g2024-03-19
- CAS:
- 348640-06-2
- Min. Order:
- 1g
- Purity:
- 98
- Supply Ability:
- 500 Kg
4-Bromo-7-azaindole

US $0.00-0.00/KG2024-02-01
- CAS:
- 348640-06-2
- Min. Order:
- 5mg
- Purity:
- 99%HPLC
- Supply Ability:
- 2000tons